One-year self-reported neurological sequelae in older COVID-19 survivors
Abstract
Aim: With the increasing number of patients recovered from COVID-19, the long-term health consequences of this disease have attracted much attention. Neurological complications are commonly seen in the acute phase of COVID-19, especially in older adults. This study aimed to investigate the long-term neurological sequelae in older COVID-19 survivors.
Methods: A total of 1438 COVID-19 survivors were recruited in this study. One year after hospital discharge, information about self-reported symptoms of the central and peripheral nervous system was collected. Comparisons of these neurological symptoms between COVID-19 survivors with severe and nonsevere cases were performed.
Results: A total of 139 (53.46%) COVID-19 survivors with severe cases and 328 (27.84%) survivors with nonsevere cases reported at least one neurological symptom one year after discharge. Most of these neurological symptoms were symptoms of the central nervous system. Specifically, 126 (48.46%) survivors with severe cases and 306 (25.98%) survivors with nonsevere cases reported at least one CNS symptom. The most frequently reported symptoms were memory deficit [234 (16.27%)] and attention deficit [80 (5.56%)]. Disease severity was associated with increased risks of long-term neurological sequelae of COVID-19.
Conclusion: This study demonstrated that neurological sequelae of COVID-19 are common one year after patient discharge, suggesting that the effects of COVID-19 on the neurological system are prolonged.
Keywords
INTRODUCTION
COVID-19 has infected over 4 billion people worldwide and the number is increasing. With the increasing number of patients who recovered from COVID-19, the long-term health consequences of COVID-19 have attracted much attention[1]. Neurological manifestations of COVID-19 are commonly observed in the acute phase of the disease, including symptoms of the central and/or peripheral nervous system[2]. Neurological sequelae such as headache, dizziness, movement disorders, and attention deficits were observed, and these symptoms are more prevalent in survivors with severe cases[3,4]. We have previously demonstrated that COVID-19 had long-term effects on the cognitive performances of older survivors, especially those who survived severe cases[1,5]. Therefore, it is more urgent to demonstrate the neurological sequelae of COVID-19 in older adults, especially survivors of severe cases. This study aims to investigate the self-reported neurological sequelae of COVID-19 one year after patient discharge.
METHODS
Subjects
This cross-sectional study was conducted one year after patient discharge, which included 1438 COVID-19 survivors aged 60 years or above. These subjects were discharged from three COVID-19 designated hospitals in Wuhan, China, from February 10 to April 10, 2020. Among these subjects, 260 were severe cases and 1178 were nonsevere cases. This study was conducted simultaneously with our previous reports, which aimed to determine the long-term effects of COVID-19 on cognition in older hospital survivors[1,5]. Therefore, the inclusion and exclusion criteria were consistent with these two publications.
The research protocols were approved by the institutional review boards of Daping Hospital, as the medical staff of this hospital worked in the COVID-19-designated Huoshenshan Hospital and Tongji Taikang Hospital, which were dismissed after the pandemic. Since this study was conducted based on telephone interviews, the requirement for written informed consent was waived, but verbal informed consent was obtained from all participants or their legal guardians. The findings of this study were reported following the Strengthening the Reporting of Observational Studies in Epidemiology Checklist (STROBE) for cohort studies.
Clinical examinations
The demographic information, including age and sex, and clinical characteristics, including body mass index (BMI) and coexisting disorders, including hypertension, diabetes, hyperlipidemia, stroke history, coronary heart disease, and chronic obstructive pulmonary disease (COPD), the treatment during hospitalization, such as intensive care unit (ICU) admission, mechanical ventilation, high flow oxygen therapy, length of hospital stay, antiviral therapy, antibacterial therapy, intravenous globulin (IVIg) use, and glucocorticoid use, were collected from the medical records.
The diagnosis of COVID-19 was made based on the World Health Organization interim guidance[6]. The severity of COVID-19 was defined as severe or nonsevere following the American Thoracic Society (ATS) guidelines for community-acquired pneumonia[7]. Accordingly, severe cases with COVID-19 were defined as confirmed SARS-CoV-2 infection plus one of the following conditions: respiratory rate > 30 breaths/min, severe respiratory distress, or SpO2 < 90% on room air. SARS-CoV-2 infection was confirmed by high-throughput sequencing or real-time reverse-transcriptase polymerase-chain-reaction assays of nasal and pharyngeal swab specimens.
Participants were interviewed by telephone and were asked to report their neurological manifestations one year after hospital discharge. These symptoms were classified into sequelae of the central nervous system, including dizziness, headache, memory deficit, attention deficit, ataxia, and seizure, and those of the peripheral nervous system, including taste problem, smell problem, vision problem, nerve pain, and myalgia [Supplementary Table 1].
Statistical analysis
The demographic and clinical characteristics of participants were presented as medians (IQRs) for continuous variables and absolute values along with percentages for categorical variables. For the comparison of demographic and clinical characteristics among groups, the Kruskal-Wallis test, χ2 test, Fisher’s exact test, or Mann-Whitney U test was used where appropriate.
Logistic regression models were used to explore risk factors associated with neurological symptoms one year after discharge, adjusting for age, sex, BMI, and coexisting disorders. Statistical analyses were conducted using SPSS statistical package version 25 (IBM SPSS Statistics for Windows, Armonk, NY, USA) and R software version 3.6.2 (R Foundation for Statistical Computing).
RESULTS
Demographic characteristics of participants
This study included 1178 COVID-19 survivors with nonsevere cases and 260 survivors with severe cases. Severe cases were older than nonsevere cases [median (IQR): 71 (67, 79) vs. 68 (66, 73), P < 0.001]. Severe cases had lower education levels [median (IQR): 12 (6, 12) vs. 12 (9, 12), P = 0.05] and higher BMI [median (IQR): 24.38 (22.90, 25.64) vs. 23.93 (22.44, 25.33), P = 0.009] than nonsevere cases. Severe cases had higher proportion of subjects with hypertension [number (%): 133 (51.15) vs. 426 (36.16), P < 0.001], diabetes [number (%): 65 (25.00) vs. 208 (17.66), P = 0.01], stroke history [number (%): 42 (16.15) vs. 37 (3.14), P < 0.001], coronary heart disease [number (%): 71 (27.31) vs. 121 (10.27), P < 0.001] and COPD [number (%): 43 (16.38) vs. 99 (8.40), P < 0.001]. As expected, severe cases had higher proportion of subjects received ICU treatment [number (%): 72 (27.69) vs. 0, P < 0.001], mechanical ventilation [number (%): 83 (31.92) vs. 0, P < 0.001], high flow oxygen therapy [number (%): 106 (40.77) vs. 184 (15.62), P < 0.001], delirium [number (%): 82 (31.54) vs. 10 (0.85), P < 0.001], and had longer length of hospital stay [median (IQR): 28 (22, 34) vs. 19 (14, 23), P < 0.001]. Furthermore, severe cases had higher proportion of subjects who received antibacterial therapy [number (%): 143 (55.00) vs. 131 (11.12), P < 0.001], IVIg treatment [number (%): 143 (55.00) vs. 22 (1.87), P < 0.001] and glucocorticoid treatment [number (%): 144 (55.38 vs. 152 (12.90), P < 0.001] than nonsevere cases [Table 1].
Demographic and baseline information of participants
| Total group (n = 1438) | Severe cases (n = 260) | Nonsevere cases (n = 1178) | P value | |
| Age - Median (IQR), year | 69 (66, 74) | 71 (67, 79) | 68 (66, 73) | < 0.001a |
| Male - No. (%) | 691 (48.05) | 133 (51.15) | 557 (47.28) | 0.27b |
| Education - Median (IQR), year | 12 (9, 12) | 12 (6, 12) | 12 (9, 12) | 0.05a |
| BMI - Median (IQR), kg/m2 | 23.99 (22.54, 25.38) | 24.38 (22.90, 25.64) | 23.93 (22.44, 25.33) | 0.009a |
| Coexisting disorders - No. (%) | ||||
| Hypertension | 561 (39.01) | 133 (51.15) | 426 (36.16) | < 0.001b |
| Diabetes mellitus | 274 (19.05) | 65 (25.00) | 208 (17.66) | 0.01b |
| Hyperlipidaemia | 142 (9.87) | 31 (11.92) | 111 (9.42) | 0.25b |
| Stroke history | 79 (5.49) | 42 (16.15) | 37 (3.14) | < 0.001b |
| Coronary heart disease | 193 (13.42) | 71 (27.31) | 121 (10.27) | < 0.001b |
| COPD | 142 (9.87) | 43 (16.38) | 99 (8.40) | < 0.001b |
| ICU admission - No. (%) | 72 (5.01) | 72 (27.69) | 0 (0) | < 0.001b |
| Mechanical ventilation, No. (%) | 83 (5.77) | 83 (31.92) | 0 (0) | < 0.001b |
| High flow oxygen therapy, No. (%) | 290 (20.17) | 106 (40.77) | 184 (15.62) | < 0.001b |
| Delirium, No. (%) | 92 (6.40) | 82 (31.54) | 10 (0.85) | < 0.001b |
| Length of hospital stay (IQR), day | 20 (15, 25) | 28 (22, 34) | 19 (14, 23) | < 0.001a |
| Antiviral therapy - No. (%) | 1107 (76.98) | 209 (80.38) | 898 (76.23) | 0.17b |
| Lianhua Qingwen | 703 (48.89) | 136 (52.31) | 567 (48.13) | 0.24b |
| Arbidol | 530 (36.86) | 106 (40.77) | 424 (35.99) | 0.16b |
| Kaletra | 125 (8.69) | 28 (10.77) | 97 (8.23) | 0.18b |
| Oseltamivir | 52 (3.62) | 10 (3.85) | 42 (3.57) | 0.85b |
| Ribavirin | 9 (0.63) | 2 (0.77) | 7 (0.59) | 0.67b |
| Other antiviral drugs | 20 (1.39) | 3 (1.15) | 17 (1.44) | 1.00b |
| Antibacterial therapy - No. (%) | 274 (19.05) | 143 (55.00) | 131 (11.12) | < 0.001b |
| IVIg treatment - No. (%) | 165 (11.47) | 143 (55.00) | 22 (1.87) | < 0.001b |
| Glucocorticoid - No. (%) | 296 (20.58) | 144 (55.38) | 152 (12.90) | < 0.001b |
Neurological sequelae of COVID-19 survivors
One year after patient discharge, 467 (32.48%) survivors reported at least one neurological symptom. Specifically, 432 (30.04%) survivors reported at least one symptom of the central nervous system, and 45 (3.13%) survivors reported at least one symptom of the peripheral nervous system. The most-reported symptom was memory loss [234 (16.27%)], followed by dizziness [96 (6.68%)], headache [80 (5.56%)], attention deficit [80 (5.56%)], smell loss [16 (1.11%)], and taste loss [15 (1.04%)].
Severe and nonsevere cases had similar frequencies in dizziness [number (%): 20 (7.69) vs. 76 (6.45), P = 0.49], ataxia [number (%): 2 (0.77) vs. 1 (0.08), P = 0.09], taste problem [number (%): 5 (1.92) vs. 10 (0.85), P = 0.17], nerve pain [number (%): 2 (0.77) vs. 1 (0.08), P = 0.09] and myalgia [number (%): 4 (1.54) vs. 5 (0.42), P = 0.06]. Severe cases had higher proportion of subjects with headache [number (%): 27 (10.38) vs. 53 (4.50), P < 0.001], memory problem [number (%): 72 (27.69) vs. 162 (13.75), P < 0.001], attention deficit [number (%): 28 (10.77) vs. 52 (4.41), P < 0.001], seizure [number (%): 2 (0.77) vs. 0, P = 0.03], smell loss [number (%): 8 (3.08) vs. 8 (0.68), P = 0.003] and vision problem [number (%): 2 (0.77) vs. 0, P = 0.03] [Table 2].
Neurological sequelae one year after discharge in COVID-19 survivors
| Total group (n = 1438) | Severe cases (n = 260) | Nonsevere cases (n = 1178) | P value | |
| Any - No. (%) | 467 (32.48) | 139 (53.46) | 328 (27.84) | < 0.001 |
| CNS sequelae | ||||
| Any - No. (%) | 432 (30.04) | 126 (48.46) | 306 (25.98) | < 0.001 |
| Dizziness - No. (%) | 96 (6.68) | 20 (7.69) | 76 (6.45) | 0.49 |
| Headache - No. (%) | 80 (5.56) | 27 (10.38) | 53 (4.50) | < 0.001 |
| Memory deficit - No. (%) | 234 (16.27) | 72 (27.69) | 162 (13.75) | < 0.001 |
| Attention deficit - No. (%) | 80 (5.56) | 28 (10.77) | 52 (4.41) | < 0.001 |
| Ataxia - No. (%) | 3 (0.21) | 2 (0.77) | 1 (0.08) | 0.09 |
| Seizure - No. (%) | 2 (0.14) | 2 (0.77) | 0 (0) | 0.03 |
| PNS sequelae | ||||
| Any - No. (%) | 45 (3.13) | 21 (8.08) | 24 (2.04) | < 0.001 |
| Taste problem - No. (%) | 15 (1.04) | 5 (1.92) | 10 (0.85) | 0.17 |
| Smell disorder - No. (%) | 16 (1.11) | 8 (3.08) | 8 (0.68) | 0.003 |
| Vision problem - No. (%) | 2 (0.14) | 2 (0.77) | 0 (0) | 0.03 |
| Nerve pain - No. (%) | 3 (0.21) | 2 (0.77) | 1 (0.08) | 0.09 |
| Myalgia - No. (%) | 9 (0.63) | 4 (1.54) | 5 (0.42) | 0.06 |
Associations between disease severity and neurological sequelae of COVID-19
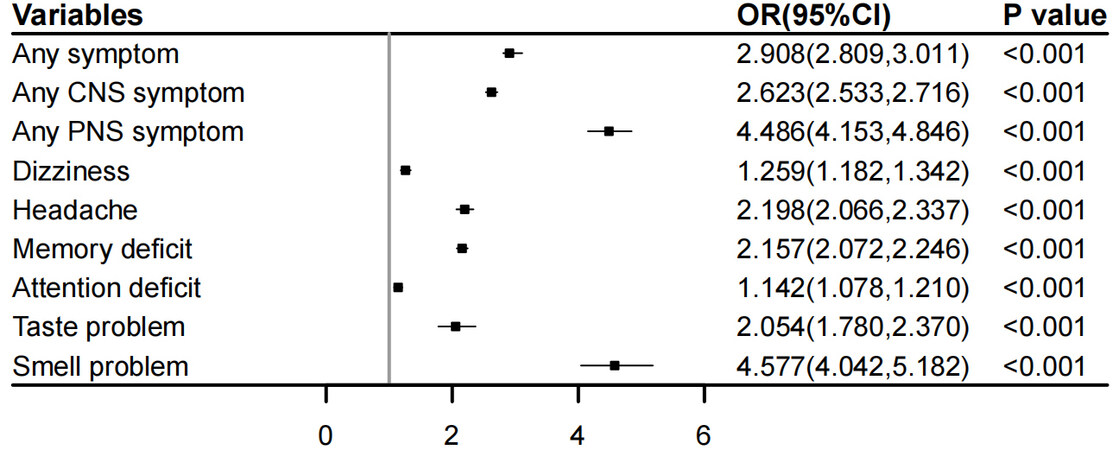
In this study, we investigated the associations between disease severity and neurological sequelae of COVID-19. We found that severe disease was associated with a higher risk of any neurological symptom [OR (95%CI): 2.908 (2.809, 3.011)], any CNS symptom [OR (95%CI): 2.623 (2.533, 2.716)] and PNS symptom [OR (95%CI): 4.486 (4.153, 4.846)]. Furthermore, severe disease was associated with a higher risk of almost all the symptoms, including dizziness [OR (95%CI): 1.259 (1.182, 1.342)], headache [OR (95%CI): 2.198 (2.066, 2.337)], memory loss [OR (95%CI): 2.157 (2.072, 2.246)], attention deficit [OR (95%CI): 1.142 (1.078, 1.210)], taste loss [OR (95%CI): 2.054 (1.780, 2.370)] and smell loss [OR (95%CI): 4.577 (4.042, 5.182)] [Figure 1]. Notably, these associations remained significant after adjusting for age, sex, BMI, and coexisting disorders, including hypertension, diabetes, hyperlipidemia, stroke, coronary heart disease, and COPD, suggesting that severe disease had a significant impact on postinfection neurological sequelae beyond these factors.
DISCUSSION
In this study, we investigated the self-reported neurological symptoms of COVID-19 in a cohort of older survivors one year after discharge. Overall, older COVID-19 survivors, especially those who survived severe cases, had a high burden of neurological sequelae. Symptoms of the central nervous system were more common than those of the peripheral nervous system. The most-reported symptom was memory loss, followed by attention deficit and dizziness.
Neurological symptoms are common in both the acute phase and post-acute phase of COVID-19[8]. The neurological sequelae of COVID-19 include a variety of symptoms, such as smell problems, taste problems, and memory deficit[9,10]. COVID-19 also increases the risk of a panel of neurological diseases, such as stroke[11] and autoimmune diseases[12]. We have recently reported that older COVID-19 survivors had an increased risk of longitudinal cognitive decline and that severe cases had a higher speed of cognitive decline than nonsevere survivors and uninfected subjects[1], which is consistent with the present findings that memory deficit was reported by 16.27% of older COVID-19 survivors. The postinfection memory deficit might be attributed to the fact that older adults are at a higher risk of cognitive impairment and that the postinfection low-grade inflammation or hypoxia status would further exacerbate long-term cognitive impairment.
Other symptoms, such as attention deficit, dizziness, and headache, have also been reported months after COVID-19 infection. It is suggested that attention deficits are very commonly seen in COVID-19 survivors[13]. This sequela of COVID-19 would dramatically impact the living quality and work efficiency of survivors. The possible association between COVID-19 and attention deficit might be attributed to the altered neurotransmitter secretion profile after SARS-CoV-2 infection[14]. Headache and dizziness after COVID-19 have also been reported by other studies[9]. However, we found in this study that symptoms of the periphery nervous system were rarely reported in COVID-19 survivors, suggesting that symptoms such as smell and taste problems could be reversible and more attention should be focused on the long-term impact of COVID-19 on the central nervous system. This might also be because our study did not utilize objective measures to determine the smell and taste of participants.
The common mechanism of postinfection neurological symptoms might be multifactual. Evidence regarding the direct invasion of the virus into the brain was limited. The most possible contributions of COVID-19 to postinfection neurological sequelae might be associated with chronic inflammatory[15,16] and long-term hypoxia status after COVID-19[17]. Other pathways by which COVID-19 insults the brain also exist. For example, recent studies have found that postinfection autoimmunity may damage neurons[18]. Neuronal reactive autoantibodies were found in COVID-19 survivors, and these autoantibodies were associated with the neurological symptoms and neuronal damage biomarkers of patients[19], suggesting an autoimmune element of neurological insult of COVID-19. Studies found that the incidence of autoimmune diseases such as Guillain-Barré syndrome[20] and multiple sclerosis[21] is increased after SARS-CoV-2 infection, further supporting this notion. Furthermore, SARS-CoV-2 is suggested to induce a wide variety of transcriptome changes in the brain regions that were associated with cognition and memory[22,23]. Neurodegenerative biomarkers are altered in biofluid of COVID-19 survivors[24]. Besides, COVID-19 is associated with mental health outcomes such as depression and anxiety, which could contribute to increased self-reported symptoms[25].
This study has several limitations. First, the symptoms were self-reported by survivors, and no objective measures were used. Second, this study only included older adults; thus, it is not clear whether younger survivors had similar sequelae. Third, this study did not include a control group with other viral infectious diseases; thus, it could not be determined whether COVID-19 had a greater long-term impact on the neurological system than other infectious diseases. This study is limited by its cross-sectional nature and no longitudinal cohort investigations were involved; therefore, it cannot be known whether these symptoms were reversible. However, this study added novel information about the long-COVID syndrome.
DECLARATIONS
Authors’ contributionsDesigned this study and drafted the manuscript: Wang LR, Yang Y
Conducted the interviews: Jiang L, Liu XY, Yan XQ
Had critical reading of the manuscript: Liu YH, Wang YJ
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateThe study was conducted in strict accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the Chinese People’s Liberation Army Specialty Medical Center. Since this study was conducted based on telephone interviews, the requirement for written informed consent was waived, but verbal informed consent was obtained from all participants or their legal guardians.
Consent for publicationAll participants agree to publication.
Copyright© The Author(s) 2022.
Supplementary MaterialsREFERENCES
1. Liu YH, Chen Y, Wang QH, et al. One-year trajectory of cognitive changes in older survivors of COVID-19 in Wuhan, China: a longitudinal cohort study. JAMA Neurol 2022;79:509-17.
2. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 2020;77:683-90.
3. Huang L, Yao Q, Gu X, et al. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. The Lancet 2021;398:747-58.
4. Heesakkers H, van der Hoeven JG, Corsten S, et al. Clinical outcomes among patients with 1-year survival following intensive care unit treatment for COVID-19. JAMA 2022;327:559-65.
5. Liu YH, Wang YR, Wang QH, et al. Post-infection cognitive impairments in a cohort of elderly patients with COVID-19. Mol Neurodegener 2021;16:48.
6. WHO. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected; 2020. Available from: https://www.scirp.org/reference/referencespapers.aspx?referenceid=2720149 [Last accessed on 30 Jun 2022].
7. Guan WJ, Ni ZY, Hu Y, et al. China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708-20.
8. Odozor CU, Kannampallil T, Ben Abdallah A, et al. Post-acute sensory neurological sequelae in patients with severe acute respiratory syndrome coronavirus 2 infection: the COVID-PN observational cohort study. Pain 2022; doi: 10.1097/j.pain.0000000000002639.
9. Fernández-de-Las-Peñas C, Navarro-Santana M, Gómez-Mayordomo V, et al. Headache as an acute and post-COVID-19 symptom in COVID-19 survivors: A meta-analysis of the current literature. Eur J Neurol 2021;28:3820-5.
10. Silva Andrade B, Siqueira S, de Assis Soares WR, et al. Long-COVID and post-COVID health complications: an up-to-date review on clinical conditions and their possible molecular mechanisms. Viruses 2021;13:700.
11. Merkler AE, Parikh NS, Mir S, et al. Risk of ischemic stroke in patients with coronavirus disease 2019 (COVID-19) vs. patients with influenza. JAMA Neurol 2020; doi: 10.1001/jamaneurol.2020.2730.
12. Luijten LWG, Leonhard SE, van der Eijk AA, et al. IGOS Consortium. Guillain-Barré syndrome after SARS-CoV-2 infection in an international prospective cohort study. Brain 2021;144:3392-404.
13. Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep 2021;11:16144.
14. Eroğlu İ, Eroğlu BÇ, Güven GS. Altered tryptophan absorption and metabolism could underlie long-term symptoms in survivors of coronavirus disease 2019 (COVID-19). Nutrition 2021;90:111308.
16. Carfì A, Bernabei R, Landi F. for the Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA 2020;324:603-5.
17. Xiong Q, Xu M, Li J, et al. Clinical sequelae of COVID-19 survivors in Wuhan, China: a single-centre longitudinal study. Clin Microbiol Infect 2021;27:89-95.
18. Franke C, Ferse C, Kreye J, et al. High frequency of cerebrospinal fluid autoantibodies in COVID-19 patients with neurological symptoms. Brain Behav Immun 2021;93:415-9.
19. Virhammar J, Nääs A, Fällmar D, et al. Biomarkers for central nervous system injury in cerebrospinal fluid are elevated in COVID-19 and associated with neurological symptoms and disease severity. Eur J Neurol 2021;28:3324-31.
20. Tard C, Maurage CA, de Paula AM, et al. Intensive Care Unit Medical Group. Anti-pan-neurofascin IgM in COVID-19-related Guillain-Barré syndrome: evidence for a nodo-paranodopathy. Neurophysiol Clin 2020;50:397-9.
21. Finsterer J. SARS-CoV-2 triggered relapse of multiple sclerosis. Clin Neurol Neurosurg 2022;215:107210.
22. Guedj E, Million M, Dudouet P, et al. 18F-FDG brain PET hypometabolism in post-SARS-CoV-2 infection: substrate for persistent/delayed disorders? Eur J Nucl Med Mol Imaging 2021;48:592-5.
23. Reiken S, Sittenfeld L, Dridi H, Liu Y, Liu X, Marks AR. Alzheimer’s-like signaling in brains of COVID-19 patients. Alzheimers Dement 2022;18:955-65.
24. Frontera JA, Boutajangout A, Masurkar AV, et al. Comparison of serum neurodegenerative biomarkers among hospitalized COVID-19 patients versus non-COVID subjects with normal cognition, mild cognitive impairment, or Alzheimer's dementia. Alzheimers Dement 2022;18:899-910.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Jiang L, Liu XY, Yan XQ, Liu YH, Wang YJ, Yang Y, Wang LR. One-year self-reported neurological sequelae in older COVID-19 survivors. Ageing Neur Dis 2022;2:10. http://dx.doi.org/10.20517/and.2022.10
AMA Style
Jiang L, Liu XY, Yan XQ, Liu YH, Wang YJ, Yang Y, Wang LR. One-year self-reported neurological sequelae in older COVID-19 survivors. Ageing and Neurodegenerative Diseases. 2022; 2(3): 10. http://dx.doi.org/10.20517/and.2022.10
Chicago/Turabian Style
Jiang, Li, Xiao-Yu Liu, Xiao-Qin Yan, Yu-Hui Liu, Yan-Jiang Wang, Ying Yang, Ling-Ru Wang. 2022. "One-year self-reported neurological sequelae in older COVID-19 survivors" Ageing and Neurodegenerative Diseases. 2, no.3: 10. http://dx.doi.org/10.20517/and.2022.10
ACS Style
Jiang, L.; Liu X.Y.; Yan X.Q.; Liu Y.H.; Wang Y.J.; Yang Y.; Wang L.R. One-year self-reported neurological sequelae in older COVID-19 survivors. Ageing. Neur. Dis. 2022, 2, 10. http://dx.doi.org/10.20517/and.2022.10
About This Article
Copyright
Data & Comments
Data
 Cite This Article 18 clicks
Cite This Article 18 clicks













Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.