New articles from Prof. David Rubinsztein, our Honorary Editors-in-Chief
In this issue, we will share five articles from Professor David Rubinsztein, our Honorary Editors-in-Chief.
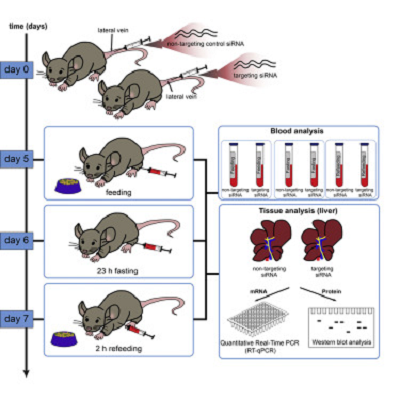
Title: Transient siRNA-mediated protein knockdown in mouse followed by feeding/starving cycle and liver tissue analysis
Authors: LidiaWrobel, Farah H.Siddiqi, David C.Rubinsztein
Type: Protocol from STAR Protocols
Highlights:
• siRNA-mediated knockdown of a gene of interest in mouse liver
• Gene expression analysis at the mRNA and protein levels in the liver tissue
• Fasting/refeeding response at the mRNA and protein levels in the liver tissue
Summary:
We present a protocol for in vivo siRNA-mediated knockdown of a gene of interest in mouse liver using systemic delivery via intravenous injection. We describe a step-by-step protocol for delivery of siRNA particles, with tips on how to optimize dosage. We detail steps for feeding/starving cycles as well as for liver tissue isolation, followed by gene expression analysis, measured at the mRNA and protein levels.
Graphical abstract:
Access this article: https://doi.org/10.1016/j.xpro.2021.100500
Title: α-Catenin levels determine direction of YAP/TAZ response to autophagy perturbation
Authors: Mariana Pavel, So Jung Park, Rebecca A. Frake, Sung Min Son, Marco M. Manni, Carla F. Bento, Maurizio Renna, Thomas Ricketts, Fiona M. Menzies, Radu Tanasa & David C. Rubinsztein
Type: Article from Nature Communications
Abstract:
The factors regulating cellular identity are critical for understanding the transition from health to disease and responses to therapies. Recent literature suggests that autophagy compromise may cause opposite effects in different contexts by either activating or inhibiting YAP/TAZ co-transcriptional regulators of the Hippo pathway via unrelated mechanisms. Here, we confirm that autophagy perturbation in different cell types can cause opposite responses in growth-promoting oncogenic YAP/TAZ transcriptional signalling. These apparently contradictory responses can be resolved by a feedback loop where autophagy negatively regulates the levels of α-catenins, LC3-interacting proteins that inhibit YAP/TAZ, which, in turn, positively regulate autophagy. High basal levels of α-catenins enable autophagy induction to positively regulate YAP/TAZ, while low α-catenins cause YAP/TAZ activation upon autophagy inhibition. These data reveal how feedback loops enable post-transcriptional determination of cell identity and how levels of a single intermediary protein can dictate the direction of response to external or internal perturbations.
Access this article: https://doi.org/10.1038/s41467-021-21882-1
Title: Autophagy regulation by acetylation—implications for neurodegenerative diseases
Authors: Sung Min Son, So Jung Park, Marian Fernandez-Estevez & David C. Rubinsztein
Type: Review Article from Experimental & Molecular Medicine
Abstract:
Posttranslational modifications of proteins, such as acetylation, are essential for the regulation of diverse physiological processes, including metabolism, development and aging. Autophagy is an evolutionarily conserved catabolic process that involves the highly regulated sequestration of intracytoplasmic contents in double-membrane vesicles called autophagosomes, which are subsequently degraded after fusing with lysosomes. The roles and mechanisms of acetylation in autophagy control have emerged only in the last few years. In this review, we describe key molecular mechanisms by which previously identified acetyltransferases and deacetylases regulate autophagy. We highlight how p300 acetyltransferase controls mTORC1 activity to regulate autophagy under starvation and refeeding conditions in many cell types. Finally, we discuss how altered acetylation may impact various neurodegenerative diseases in which many of the causative proteins are autophagy substrates. These studies highlight some of the complexities that may need to be considered by anyone aiming to perturb acetylation under these conditions.
Access this article: https://doi.org/10.1038/s12276-021-00556-4
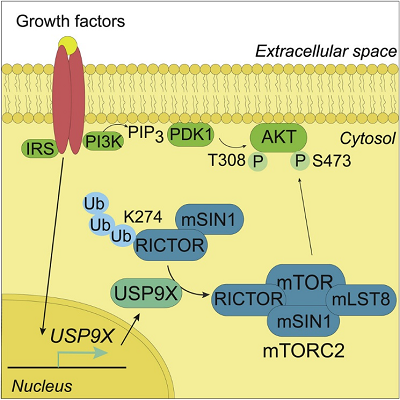
Title: mTORC2 Assembly Is Regulated by USP9X-Mediated Deubiquitination of RICTOR
Authors: Lidia Wrobel, Farah H. Siddiqi, Sandra M. Hill, Sung Min Son, Cansu Karabiyik, Hyunjeong Kim, David C. Rubinsztein
Type: Report from Cell Reports
Highlights:
• USP9X depletion decreases mTORC2 signaling through RICTOR
• RICTOR ubiquitination regulates the RICTOR-mTOR interaction
• Growth factors regulate USP9X expression to regulate hepatic mTORC2 signaling
Summary:
The mechanistic target of rapamycin complex 2 (mTORC2) controls cell metabolism and survival in response to environmental inputs. Dysregulation of mTORC2 signaling has been linked to diverse human diseases, including cancer and metabolic disorders, highlighting the importance of a tightly controlled mTORC2. While mTORC2 assembly is a critical determinant of its activity, the factors regulating this event are not well understood, and it is unclear whether this process is regulated by growth factors. Here, we present data, from human cell lines and mice, describing a mechanism by which growth factors regulate ubiquitin-specific protease 9X (USP9X) deubiquitinase to stimulate mTORC2 assembly and activity. USP9X removes Lys63-linked ubiquitin from RICTOR to promote its interaction with mTOR, thereby facilitating mTORC2 signaling. As mTORC2 is central for cellular homeostasis, understanding the mechanisms regulating mTORC2 activation toward its downstream targets is vital for our understanding of physiological processes and for developing new therapeutic strategies in pathology.
Graphical abstract:
Access this article: https://doi.org/10.1016/j.celrep.2020.108564
Title: Leucine regulates autophagy via acetylation of the mTORC1 component raptor
Authors: Sung Min Son, So Jung Park, Eleanna Stamatakou, Mariella Vicinanza, Fiona M. Menzies & David C. Rubinsztein
Type: Article from Nature Communications
Abstract:
Macroautophagy ("autophagy") is the main lysosomal catabolic process that becomes activated under nutrient-depleted conditions, like amino acid (AA) starvation. The mechanistic target of rapamycin complex 1 (mTORC1) is a well-conserved negative regulator of autophagy. While leucine (Leu) is a critical mTORC1 regulator under AA-starved conditions, how Leu regulates autophagy is poorly understood. Here, we describe that in most cell types, including neurons, Leu negatively regulates autophagosome biogenesis via its metabolite, acetyl-coenzyme A (AcCoA). AcCoA inhibits autophagy by enhancing EP300-dependent acetylation of the mTORC1 component raptor, with consequent activation of mTORC1. Interestingly, in Leu deprivation conditions, the dominant effects on autophagy are mediated by decreased raptor acetylation causing mTORC1 inhibition, rather than by altered acetylation of other autophagy regulators. Thus, in most cell types we examined, Leu regulates autophagy via the impact of its metabolite AcCoA on mTORC1, suggesting that AcCoA and EP300 play pivotal roles in cell anabolism and catabolism.
Access this article: https://doi.org/10.1038/s41467-020-16886-2






