Ferroptotic cells augment T-cell activation and neuroinflammation
Abstract
Since ferroptosis, a form of cell death characterized by aberrant lipid peroxidation, was proposed 10 years ago, its interaction with the immune system has been revealed gradually. On the one hand, immune cell-secreted cytokines are able to increase or suppress ferroptosis sensitivities of other cell types, such as tumor cells and fibroblasts. On the other hand, ferroptotic cell-released factors have the capacity to modulate the functions of neighboring immune cells, including dendritic cells, macrophages, and T cells. Identifying these immunomodulatory molecules generated during ferroptosis paves the way for developing novel immunotherapy strategies for treating cancer and autoimmune diseases.
Keywords
Ferroptosis is a form of regulated cell death triggered by unrestricted accumulation of lethal lipid peroxides on cell membranes[1]. Since it was identified 10 years ago, ferroptosis has been shown to be involved in the occurrence or progression of various pathological diseases, including cancer, neurodegeneration, cardiovascular diseases, and acute kidney injury[2,3]. The majority of previous studies have focused on illustrating the cellular intrinsic signaling and metabolic pathways that initiate or prevent the execution of ferroptosis, as well as attempted to clarify the associations between these pathways and cell death-related pathological phenotypes. However, it remains unclear how ferroptotic cells interplay with surrounding cells including immune cells in the pathological tissue microenvironment and whether their interactions contribute to the pathological progression.
Experimental autoimmune encephalitis (EAE) is the most commonly used animal model for multiple sclerosis (MS), which is an autoimmune disease characterized by inflammatory demyelination, oligodendrocyte death, and neuronal degeneration in the central nervous system[4]. MS lesions are mediated by the invasion of immune cells, including CD4+ T cells and monocytes[5]. Although certain features of ferroptosis have been observed in the MS and EAE, including iron overload, reduced expression of glutathione peroxidase-4, and oxidative damage[6], whether ferroptotic cells are really present and involved in the demyelination and MS pathogenesis is still inconclusive. Recently, Luoqian et al. observed the accumulations of iron and lipid peroxidation in the cortical tissue of EAE mice and found ferroptosis inhibitor liproxstatin-1 could relieve demyelination and neurodegeneration in animals[7]. As an essential gene for ferroptosis execution, acyl-CoA synthetase long-chain family member 4 (ACSL4) was also found to be increased in NeuN+ neuron cells along the progression of EAE. Knockdown of ACSL4 in the spinal cord reduced lipid peroxidation and ameliorated EAE severity. These results demonstrate that ferroptosis is induced in spinal cords and involved in EAE development.
Myelin autoantigen-specific T cells are major initiators and mediators of MS and EAE, including CD4+ Th1 and Th17 cells[8]. Luoqian et al. found that ferroptotic lipid peroxidation was elevated before T-cell activation at the early stage of EAE[7]. Liproxstatin-1 or ACSL4 knockdown could reduce T-cell infiltration and prevent the onset of EAE. To test whether ferroptosis in neuronal cells can directly regulate T-cell function, the supernatant of ferroptotic neurons treated with classical ferroptosis inducer RSL3 or erastin was collected. When naïve CD4+ T cells were activated by anti-CD3 and anti-CD28 antibodies, these ferroptotic supernatants could enhance the secretions of IL-2 and IFNγ from T cells, suggesting that T-cell activation was augmented by certain factors from ferroptotic neurons. Furthermore, the adoptive transfer of T cells that were pretreated by ferroptotic supernatant exacerbated EAE pathogenesis.
Finally, the authors used ceruloplasmin (Cp), a cuproenzyme that can oxidize ferrous iron into ferric iron, to prevent ferroptosis in EAE mice. Cp administration reduced the contents of iron and lipid peroxidases in the spinal cord and decreased the infiltration of CD4+ T cells, resulting in relieved demyelination, neuronal death, and attenuated EAE clinical scores.
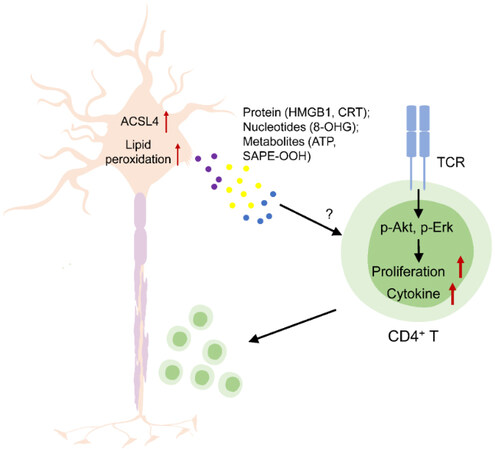
Overall, the study by Luoqian et al. demonstrated that ACSL4-mediated ferroptosis is induced in neuronal cells during the early stage of EAE progression, and then ferroptotic neurons release certain factors to augment T-cell activation and its effector function, which can accelerate the progression of EAE[7] [Figure 1]. This study is the first to provide evidence that ferroptotic cells play an immunostimulatory role by directly working on T cells, as well as enriching our understanding of how ferroptosis interplays with immune response.
Figure 1. Overview of the mechanism by which ACSL4-mediated neuronal cell ferroptosis augments CD4+ T-cell activation and EAE progression. HMGB1: High-mobility group box 1 protein; CRT: calreticulin; 8-OHG: 8-hydroxy-2’-deoxyguanosine; SAPE-OOH: 1-steaoryl-2-15-HpETE-sn-glycero-3-phosphatidylethanolamine; TCR: T-cell receptor; CD4: cluster of differentiation 4; p-Akt: phosphorylated protein kinase B; p-Erk: phosphorylated extracellular signal-regulated kinase; EAE: experimental autoimmune encephalitis.
Recently, the interactions between ferroptotic cells and immune cells have been revealed gradually and drawn more and more attention. On the one hand, immune cells are able to modulate ferroptosis sensitivity of other cells, such as tumor cells. The earliest study revealed that CD8+ T cells activated by cancer immunotherapy could sensitize melanoma cells to ferroptosis through secretion of IFNγ, which suppresses the expression of solute carrier family 7 member 11 (SLC7A11), resulting in limited uptake of cystine by tumor cells[9]. Furthermore, CD8+ T cell-secreted IFNγ was shown to coordinate with arachidonic acid to directly induce tumoral ferroptosis in the absence of synthetic molecules[10]. Combinations of checkpoint blockade and ferroptosis activators, such as an enzyme degrading cystine and cysteine or arachidonic acid supplementation, have synergistic antitumor activities across multiple murine tumor models[9,10]. In contrast to the ferroptosis sensitization effect of IFNγ, some inflammatory cytokines can prevent ferroptosis. Interleukin-6 was shown to inhibit ferroptosis of head and neck squamous cell carcinoma cells by JAK2/STAT3-mediated upregulation of SLC7A11[11]. Tumor necrosis factor (TNF), another T cell-secreted cytokine, was able to protect synovial fibroblasts from ferroptosis by increasing system xc- expression and cystine uptake. In the collagen-induced arthritis mouse model, a TNF blockade combined with a ferroptosis inducer synergistically initiated ferroptosis in synovial fibroblasts and attenuated arthritis progression[12]. Therefore, in different inflammatory scenarios, cytokines secreted by immune cells regulate the ferroptosis of their neighboring cells distinctly by reprogramming the metabolisms of fatty acids or amino acids.
On the other hand, ferroptotic cells can be sensed and processed by immune cells, including macrophages or dendritic cells, to modulate innate and adaptive immune responses. Ferroptosis was initially considered an immunogenic cell death (ICD), a type of cell demise that can elicit uptake of cellular components by dendritic cells (DCs) and enhance antigen presentation to T cells, resulting in the activation of antigen-specific cytotoxic T-cell response. The earliest evidence shows that ferroptotic cells could release damage-associated molecular patterns (DAMPs) such as high-mobility group box 1 protein (HMGB1) and calreticulin (CRT) exposure[13-15], which could all function as immune adjuvants to promote the activation and maturation of DCs[16]. Later, Efimova et al. reported that the early ferroptotic MCA205 cells induced by short-term treatment of RSL3 stimulated maturation of bone marrow-derived DCs and induced a vaccination-like effect in vivo in contrast to late ferroptotic cells[17]. Another recent study also used the same MCA205 cells whose ferroptosis was induced by ML162 or GPX4 knockdown; however, it drew the opposite conclusion that ferroptotic cells were not immunogenic regardless of the stage of cell death, even though they could release ATP, HMGB1, and cytokines including CXCL1 and IFNβ. Mechanistically, engulfed ferroptotic cells suppressed the expressions of pro-inflammatory genes and impaired antigen cross-presentation in DCs[18]. The above results suggest that ferroptotic tumor cells caused by different inducers may have different immunomodulatory effects due to certain unique molecules released by these cells. One of the crucial features of ferroptosis is the accumulation of lipid peroxides such as oxidized phospholipids of plasma membranes. Although an oxidized phospholipid [1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine (PAPC)] was shown to impair the differentiation and immune-stimulatory function of in vitro cultured DCs[19,20], it is unknown whether this oxidized PAPC is enriched in ferropototic cells. A recent study identified another oxidized phospholipid, 1-steaoryl-2-15-HpETE-sn-glycero-3-phosphatidylethanolamine (SAPE-OOH), which is generated during ferroptosis of leukemic cells and functions as an eat-me signal to promote phagocytosis of ferroptotic cells by macrophage[21]. In addition to oxidized lipids, oxidized nucleobases, such as 8-hydroxy-2’-deoxyguanosine (8-OHG), were also found to be released by GPX4 deficient pancreatic cancer cells. 8-OHG induced macrophage infiltration and activation via TMEM173, resulting in immunosuppression and tumor progression[22]. Therefore, although it is still controversial whether ferroptosis is immunogenic or not, specific molecules generated and released from ferroptotic cells would modulate the functions of DCs or macrophages and the subsequent engagement of T-cell response.
Furthermore, it would be worth knowing whether ferroptotic cells have direct impacts on other immune cells, especially T cells. Luoqian and colleagues provided the first evidence that some factors released from ferroptotic cells work on T cells directly to enhance their activation and effector function. These mysterious factors are present in the conditioned medium from ferroptotic primary neurons treated with RSL3 or erastin. Although the identities of these factors are not revealed yet, they have the ability to amplify the signaling transduction of T-cell receptors, including activations of Akt and Erk. These data inspire our interest in further investigating the characteristics and identities of these T cell-promoting factors, although it will be more rigorous to test the effects of trace residuals of RSL3 or erastin in the supernatant from ferroptotic cells on T cells.
Altogether, ferroptotic cells could be immunosuppressive or immunostimulatory due to their broader impacts on various types of immune cells, including DCs, macrophages, and T cells. Identifying these immunomodulatory molecules generated from ferroptotic cells will be the top priority of future research in this field. It is also worth knowing whether these ferroptosis-related immunomodulatory molecules are cell type-specific. In other words, can the same factor act on multiple types of immune cells? A further question is: Do different ferroptotic cells release different sets of immunomodulatory molecules? For example, other than neurons, can other types of ferroptotic cells release the same T cell-promoting factors? The answers to these questions hold promise for developing novel therapeutic approaches to treat cancer or autoimmune diseases.
DECLARATIONS
Authors’ contributionsConceptualization, writing-review & editing: Lu F, Wang W
Writing-original draft: Xue Y
All authors read and approved the final manuscript.
Availability of data and materialsAll data described in the article are provided within the article.
Financial support and sponsorshipThis work was supported in part by the Independent Innovation Grant from Huazhong University of Science & Technology to Wang W (grant 2021GCRC072), and grants from the National Natural Science Foundation of China to Wang W (grant 81902930, 82073177) and Lu F (grant 82102930), and the National Key R&D Program of China (grant 2021YFC2400600/2021YFC2400602).
Conflicts of interestAll authors declared that there are conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
REFERENCES
1. Dixon SJ, Lemberg KM, Lamprecht MR, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 2012;149:1060-72.
2. Angeli JPF, Shah R, Pratt DA, Conrad M. Ferroptosis inhibition: mechanisms and opportunities. Trends Pharmacol Sci 2017;38:489-98.
3. Stockwell BR, Friedmann Angeli JP, Bayir H, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell 2017;171:273-85.
5. Maugh TH 2nd. The EAE model: a tentative connection to multiple sclerosis. Science 1977;195:969-71.
6. Bagnato F, Hametner S, Yao B, et al. Tracking iron in multiple sclerosis: a combined imaging and histopathological study at 7 Tesla. Brain 2011;134:3602-15.
7. Luoqian J, Yang W, Ding X, et al. Ferroptosis promotes T-cell activation-induced neurodegeneration in multiple sclerosis. Cell Mol Immunol 2022;19:913-24.
8. Kuchroo VK, Anderson AC, Waldner H, Munder M, Bettelli E, Nicholson LB. T cell response in experimental autoimmune encephalomyelitis (EAE): role of self and cross-reactive antigens in shaping, tuning, and regulating the autopathogenic T cell repertoire. Annu Rev Immunol 2002;20:101-23.
9. Wang W, Green M, Choi JE, et al. CD8+ T cells regulate tumour ferroptosis during cancer immunotherapy. Nature 2019;569:270-4.
10. Liao P, Wang W, Wang W, et al. CD8+ T cells and fatty acids orchestrate tumor ferroptosis and immunity via ACSL4. Cancer Cell 2022;40:365-378.e6.
11. Li M, Jin S, Zhang Z, Ma H, Yang X. Interleukin-6 facilitates tumor progression by inducing ferroptosis resistance in head and neck squamous cell carcinoma. Cancer Lett 2022;527:28-40.
12. Wu J, Feng Z, Chen L, et al. TNF antagonist sensitizes synovial fibroblasts to ferroptotic cell death in collagen-induced arthritis mouse models. Nat Commun 2022;13:676.
13. Wen Q, Liu J, Kang R, Zhou B, Tang D. The release and activity of HMGB1 in ferroptosis. Biochem Biophys Res Commun 2019;510:278-83.
14. Ye F, Chai W, Xie M, et al. HMGB1 regulates erastin-induced ferroptosis via RAS-JNK/p38 signaling in HL-60/NRASQ61L cells. Am J Cancer Res 2019;9:730-9.
15. Yu B, Choi B, Li W, Kim DH. Magnetic field boosted ferroptosis-like cell death and responsive MRI using hybrid vesicles for cancer immunotherapy. Nat Commun 2020;11:3637.
16. Yamazaki T, Hannani D, Poirier-Colame V, et al. Defective immunogenic cell death of HMGB1-deficient tumors: compensatory therapy with TLR4 agonists. Cell Death Differ 2014;21:69-78.
17. Efimova I, Catanzaro E, Van der Meeren L, et al. Vaccination with early ferroptotic cancer cells induces efficient antitumor immunity. J Immunother Cancer 2020;8:e001369.
18. Wiernicki B, Maschalidi S, Pinney J, et al. Cancer cells dying from ferroptosis impede dendritic cell-mediated anti-tumor immunity. Nat Commun 2022;13:3676.
19. Blüml S, Zupkovitz G, Kirchberger S, et al. Epigenetic regulation of dendritic cell differentiation and function by oxidized phospholipids. Blood 2009;114:5481-9.
20. Blüml S, Kirchberger S, Bochkov VN, et al. Oxidized phospholipids negatively regulate dendritic cell maturation induced by TLRs and CD40. J Immunol 2005;175:501-8.
21. Luo X, Gong HB, Gao HY, et al. Oxygenated phosphatidylethanolamine navigates phagocytosis of ferroptotic cells by interacting with TLR2. Cell Death Differ 2021;28:1971-89.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Xue Y, Lu F, Wang W. Ferroptotic cells augment T-cell activation and neuroinflammation. Ageing Neur Dis 2022;2:15. http://dx.doi.org/10.20517/and.2022.17
AMA Style
Xue Y, Lu F, Wang W. Ferroptotic cells augment T-cell activation and neuroinflammation. Ageing and Neurodegenerative Diseases. 2022; 2(4): 15. http://dx.doi.org/10.20517/and.2022.17
Chicago/Turabian Style
Xue, Ying, Fujia Lu, Weimin Wang. 2022. "Ferroptotic cells augment T-cell activation and neuroinflammation" Ageing and Neurodegenerative Diseases. 2, no.4: 15. http://dx.doi.org/10.20517/and.2022.17
ACS Style
Xue, Y.; Lu F.; Wang W. Ferroptotic cells augment T-cell activation and neuroinflammation. Ageing. Neur. Dis. 2022, 2, 15. http://dx.doi.org/10.20517/and.2022.17
About This Article
Copyright
Data & Comments
Data
 Cite This Article 13 clicks
Cite This Article 13 clicks














Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.