Chronic intracerebroventricular administration is a reliable method in brain studies on monkeys
Abstract
Intracerebroventricular (ICV) administration through cannulas is a direct way to deliver large molecules and substances that are blocked by the blood-brain barrier into the central nervous system (CNS). It is widely used in brain studies on monkeys. However, this method is invasive, as it requires guide cannulas to be implanted into the brain. Whether the long-term implantation of the cannula and the administration of molecule-delivering vehicles, usually saline, can affect the brain by inducing chronic CNS inflammation or even worse brain atrophy, remains an issue to be solved. To answer this question, we investigated inflammatory markers and brain structures on three vehicle-control monkeys who received cannula implantation and one-year ICV saline administration in another study. During the experiment, the monkeys’ cerebrospinal fluid (CSF) samples were collected periodically, and the level of three classic inflammatory markers (IL-1β, IL-6, and TNF-α) were measured by electrochemiluminescence immunoassay. The monkeys’ brain structures were imaged in vivo periodically by 9.4 Tesla magnetic resonance imaging, which can provide the best-resolution magnetic resonance images of living monkeys, and the volume of the hippocampus was measured to evaluate the brain atrophy. The data reveal that, during the administrating period, the long-term levels of the inflammatory markers in the CSF and the volumes of the hippocampus did not change significantly compared with the baseline. These results suggest that the long-term ICV administration of saline through cannulas did not induce chronic neuroinflammation or brain atrophy in these rhesus monkeys, suggesting chronic ICV administration via implanted cannulas is a reliable method in monkey brain research.
Keywords
The central nervous system is protected by the blood-brain barrier (BBB), which is a semipermeable structure composed of tight endothelial junctions to provide a physical barrier for the entry of large molecules[1]. It restricts circulating toxic particles but facilitates nutrients to be delivered into the brain. Meanwhile, some therapeutic substances and test articles are also blocked by the BBB[2]. Intracerebroventricular (ICV) injection physically breaches the BBB and enables the direct administration of large molecules, including chemicals, virus vectors, and therapeutic substances, into the central nervous system (CNS) through pre-implanted guide cannulas. Studies on the distribution of injected large molecules revealed that large molecules including adenovirus vectors and proteins can spread into the subarachnoid space, meninges, and choroid plexus along with the flow of the CSF and diffuse into the brain parenchyma adjacent to these sites[3-5]. Although this method is an effective way to deliver molecules into the CNS, it is invasive and could affect the brain in many ways.
One major concern in chronic cannula-guided ICV administration comes from the implantation of the guide cannulas. To ease the repeated ICV injection, one or two guide cannulas must be implanted into the lateral ventricles. Drugs or test articles are injected into the lateral ventricles via the guide cannulas without repeated penetration of the brain parenchyma. The implantation of guide cannulas causes acute brain injury. It is well established that an acute brain injury induces acute inflammation in the brain, which is one important step in the repair of the injury. However, it is not clear if long-term implantation of cannulas can induce chronic neuroinflammation.
Chronic neuroinflammation is a persistently activated immune response in the CNS, which is always marked by sustained release of pro-inflammatory cytokines, including interleukins, tumor necrosis factor-α (TNF-α), transforming growth factor-β (TGF-β), and interferon-γ (IFN-γ)[6, 7]. Pro-inflammatory cytokines exert various functions in neuroinflammation, including induction of chemokines and adhesion molecules and recruitment of the peripheral immune cells into the CNS, leading to neuronal apoptosis and activation of the glial cells[6]. Increasing evidence suggests that cytokine-mediated neuroinflammation plays an important role in initiating or deteriorating neurodegenerative diseases[8-10]. Clinical evidence reveals that the pro-inflammatory cytokines are elevated in the body fluid of individuals with neurodegenerative diseases, including Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis[11-13]. Therefore, if cannula-based ICV administration can induce chronic neuroinflammation, the results of studies on brain diseases and mechanisms would be seriously confounded, and the application of this method would be seriously limited.
Non-human primates are phylogenetically closer to humans than rodents. They share with humans many brain regions, circuits, and neural cells, which are critical for higher cognitive capacity. Thus, monkeys are widely used in the study of brain diseases and mechanisms, including in CNS disease modeling with ICV administration[14-16]. Unfortunately, no study on whether cannula-based ICV administration can induce chronic neuroinflammation in monkeys has been reported thus far.
To investigate if long-term cannula implantation and ICV saline administration can induce chronic neuroinflammation in monkeys, the levels of three most studied pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α) in the CSF of three vehicle-control monkeys used as vehicle-control in another study were continuously monitored during one-year cannula-guide ICV saline administration [see Supplementary Materials for more method details]. The measurement of IL-1β, IL-6, and TNF-α in the CSF was carried out by an electrochemiluminescence immunoassay system, which has an extremely low detection limit in the measurement of biomarkers in body fluid.
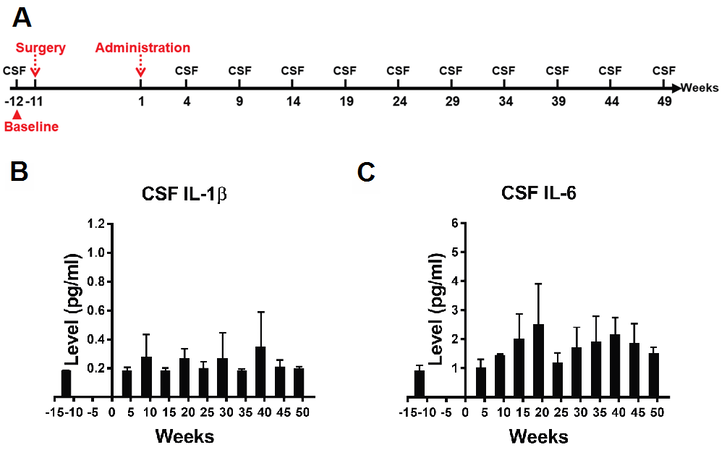
The levels of CSF IL-1β, IL-6, and TNF-α were measured at 12 weeks before the administration (the baseline) and the 4, 9, 14, 19, 24, 29, 34, and 39 weeks after the administration [Figure 1A]. Kruskal-Wallis H test revealed the effect of ICV saline administration on the average levels of IL-1β and IL-6 was not significant (P = 0.597 for IL-1β, P = 0.112 for IL-6, Figure 1B and C). The levels of CSF TNF-α were around the low detection limit, and no meaningful statistics could be applied. The data for TNF-α are not shown. In summary, the results reveal that there was no elevated CSF IL-1β, IL-6, or TNF-α during the administration, which suggests there was no significant neuroinflammation during the ICV saline administration.
Figure 1. (A) The schedule of CSF sample collection. Cannula implantation was carried out in Week 11. ICV administration began in Week 1. CSF samples were collected in Weeks 12, 4, 9, 14, 19, 24, 29, 34, 39, 44, and 49. CSF sample collected at Week -12 was taken as the baseline. (B,C) Kruskal-Wallis H test revealed no significant difference between different time points in the average levels of CSF IL-1β (P = 0.597) and IL-6 (P = 0.112). Data are presented as mean ± standard deviation (SD). CSF: Cerebrospinal fluid; ICV: intracerebroventricular.
In addition, we also investigated whether there was any brain atrophy caused by long-term cannula implantation and ICV administration, considering reports on brain atrophy induced by traumatic brain injury and neuroinflammation[17-19]. The structures of the monkeys’ brains were imaged periodically by magnetic resonance imaging (MRI) (see Supplementary Materials for more method details). To increase the accuracy in the measurement of hippocampus volumes, the monkeys’ brains were scanned by a 9.4 Tesla MRI system, which provides the highest resolution structural images currently available on living monkeys.
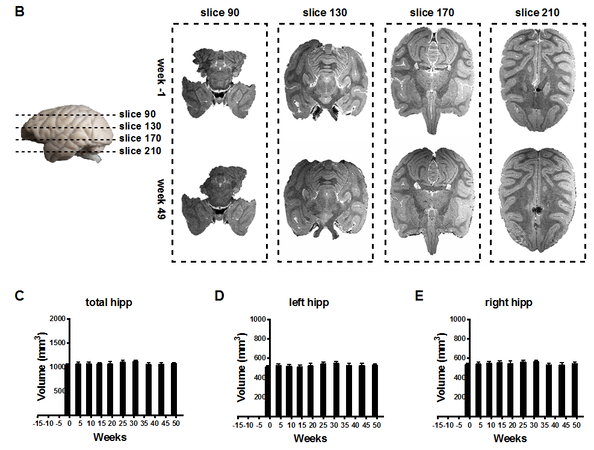
The measurement was carried out one week before the administration (the baseline) and 4, 9, 14, 19, 25, 31, 37, 43, and 49 weeks after administration [Figure 2A]. First, longitudinal MR images from the same subject were realigned to examine general structural changes in the brain. No significant brain atrophy was observed on MR images from each time point compared with the baseline [Figure 2B]. Then, the volume of the hippocampus was calculated to evaluate the atrophy in the brain because it is one of the most vulnerable structures in the brain and has a clear contour on MR images. Kruskal-Wallis H test revealed that the effect of ICV saline administration on the average volumes of total hippocampus was not significant (P = 0.775, Figure 2C). We further compared the average volumes of the left and right hippocampus independently. Kruskal-Wallis H test also revealed that the difference between each time point in the volumes of the left and right hippocampus were both not significant (P = 0.770 for the left hippocampus, P = 0.606 for the right hippocampus, Figure 2D and E). In summary, the results from the ultra-high-resolution MRI scan revealed that there was no reduction in the volume of the hippocampus, which suggests there was no significant atrophy in the brain.
Figure 2. (A) Schedule of MRI scans. Cannula implantation was carried out in Week -11. ICV administration began in Week 1. MR images were acquired in Weeks 1, 4, 9, 14, 19, 25, 31, 37, 43, and 49. MR images acquired in Week -1 were taken as the baseline. (B) Representative MR images show there was no significant brain atrophy in Week 49 when compared with the baseline (Week 11). (C-E) Kruskal-Wallis H test revealed no significant difference between different time points in the average total hippocampus volumes (P = 0.775), the average left hippocampus volumes (P = 0.770), and the average right hippocampus volumes (P = 0.606). Data are presented as mean ± standard deviation (SD). MRI: Magnetic resonance imaging; ICV: intracerebroventricular.
Here, we demonstrate that, compared with the baseline, there was no elevated IL-1β or IL-6 in the CSF of the three cannula implanted monkeys during 1-year ICV saline administration, suggesting that the cannula-guided long-term ICV administration technique did not induce chronic neuroinflammation in the monkeys. The other finding that there was no significant decrease in hippocampus volumes supports this conclusion.
To increase the accuracy of the measurement of inflammatory cytokines in the body fluid, an advanced detection method was employed in this study. Inflammatory cytokines in the body fluid exert their physiological functions at low levels, and many of them are usually below the low detection threshold under physiological conditions using common enzyme-linked immunosorbent assay kits[20]. Advanced techniques are developed to detect the cytokines in the body fluids, with much higher detection sensitivity and smaller specimen volumes. Among the newly developed assay techniques, the MSD multiplex immunoassay platform represents a combination of electrochemiluminescence and patterned arrays with an ultra-low detection limit, which is also one of the most used methods in the measurement of cytokines in body fluids nowadays. The detection sensitivity can reach 0.1 pg/mL for some cytokines using this method. The reliability of this method was validated in three recent studies, which proved that this method had a high detection rate for most of the cytokines and displayed a moderate-to-excellent intra-individual variability[21-23]. In our experiment, the detection rate was 100% for IL-1β and IL-6, but only 60% for the TNF-α, which indicates that the level of TNF-α in the CSF of monkeys under physiological conditions was extremely low.
The hippocampus is one of the most vulnerable structures in the brain under many conditions such as injury, aging, and neurodegenerative diseases[24], and it is lying in the inferior horn of the lateral ventricle, directly affected by ICV administration. Due to the vulnerability and clear contour in MR images, the hippocampus volume was taken as an indicator of brain atrophy in this study. To investigate if ICV saline administration can induce atrophy, ultra-high magnetic field MRI was employed to image the brain. The fact that we did not find any decrease in the hippocampus volumes strongly suggests no brain atrophy in the monkeys’ brains, which also supported the conclusion that there was no significant inflammation in the brain.
Taken together, our results reveal that long-term cannula implantation and ICV administration of drug-vehicle do not induce chronic neuroinflammation and brain atrophy in rhesus monkeys, which suggests that chronic ICV administration is a reliable method for brain studies on monkeys.
DECLARATIONS
AcknowledgmentsWe thank Professor Zhong Kai’s (High Magnetic Field Laboratory, Chinese Academy of Science) great help in MRI scanning and MRI data analysis. Thank Jing Wu for providing technical help in the cannula implantation surgery. Thank Hong-yi Yang, Yun Wu, and Lu-lu Wang for providing great endeavor in the MRI scanning, including the development of the head coil, and optimizing the sequence of the MRI scanning.
Author’s contributionsDesigned the experiment: Hu XT, Yin Y, Wu SH, and Zhang LH
Carried out the cannula implantation surgery: Wu J, Wang WC, Huang RY, Xu JL, and Zhang LH
Carried out the MRI scanning: Yang HY, Wu Y, Wang LL, and Zhang LH
Carried out the CSF sample collection: Tong HY, Zhang J, Zhang LH, Su J, and Luo XR
Carried out the data analysis: Zhang LH
Wrote the manuscript: Zhang LH
Revised the manuscript: Hu XT and Yin Y
All authors read and approved the final version of the manuscript.
Availability of Data and MaterialsNot applicable.
Financial Supports and SponsorshipThis work was supported by the National Program for National Key R&D Program of China (2018YFA0801403), Key Realm R&D Program of Guangdong Province (2019B030335001), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB32060200), the National Natural Science Foundation of China (81941014, 81771387, 31700897), the Applied Basic Research Programs of Science and Technology Commission Foundation of Yunnan Province (2019FA007), China Postdoctoral Science Foundation (2018M631105) and CAS “Light of West China” Program.
Conflicts of InterestAll authors declared that there are no conflicts of interests.
Ethical Approval and Consent to ParticipateAll experimental procedures were carried out following “Guide for the Care and Use of Laboratory Animals”[20] and approved by the Animal Care and Use Committee (IACUC) of the Kunming Institute of Zoology, Chinese Academy of Sciences (IACUC No.: IACUC19001).
Consent for PublicationNot applicable.
Copyright© The Author(s) 2022.
REFERENCES
2. Bauer HC, Krizbai IA, Bauer H, Traweger A. “You Shall Not Pass”-tight junctions of the blood brain barrier. Front Neurosci 2014;8:392.
3. Vuillemenot BR, Kennedy D, Reed RP, et al. Recombinant human tripeptidyl peptidase-1 infusion to the monkey CNS: safety, pharmacokinetics, and distribution. Toxicol Appl Pharmacol 2014;277:49-57.
4. Kawasaki H, Kosugi I, Sakao-Suzuki M, Meguro S, Tsutsui Y, Iwashita T. Intracerebroventricular and intravascular injection of viral particles and fluorescent microbeads into the neonatal brain. J Vis Exp 2016; doi: 10.3791/54164.
5. Driesse MJ, Kros JM, Avezaat CJ, et al. Distribution of recombinant adenovirus in the cerebrospinal fluid of nonhuman primates. Hum Gene Ther 1999;10:2347-54.
6. Lucas SM, Rothwell NJ, Gibson RM. The role of inflammation in CNS injury and disease. Br J Pharmacol 2006;147 Suppl 1:S232-40.
7. Heppner FL, Ransohoff RM, Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci 2015;16:358-72.
8. Sankowski R, Mader S, Valdés-Ferrer SI. Systemic inflammation and the brain: novel roles of genetic, molecular, and environmental cues as drivers of neurodegeneration. Front Cell Neurosci 2015;9:28.
10. Galimberti D, Fenoglio C, Scarpini E. Inflammation in neurodegenerative disorders: friend or foe? Curr Aging Sci 2008;1:30-41.
11. Mitchell RM, Freeman WM, Randazzo WT, et al. A CSF biomarker panel for identification of patients with amyotrophic lateral sclerosis. Neurology 2009;72:14-9.
12. Lindqvist D, Hall S, Surova Y, et al. Cerebrospinal fluid inflammatory markers in Parkinson’s disease - associations with depression, fatigue, and cognitive impairment. Brain Behav Immun 2013;33:183-9.
13. Darweesh SKL, Wolters FJ, Ikram MA, de Wolf F, Bos D, Hofman A. Inflammatory markers and the risk of dementia and Alzheimer’s disease: a meta-analysis. Alzheimers Dement 2018;14:1450-9.
14. Zhai R, Rizak J, Zheng N, et al. Alzheimer’s Disease-like pathologies and cognitive impairments induced by formaldehyde in non-human primates. Curr Alzheimer Res 2018;15:1304-21.
15. Li H, Lei X, Huang B, et al. A quantitative approach to developing Parkinsonian monkeys (Macaca fascicularis) with intracerebroventricular 1-methyl-4-phenylpyridinium injections. J Neurosci Methods 2015;251:99-107.
16. Erratum: Forny-Germano et al. , “Alzheimer’s Disease-like pathology induced by amyloid-β oligomers in nonhuman primates”. J Neurosci 2020;40:8204.
17. Tate DF, Bigler ED. Fornix and hippocampal atrophy in traumatic brain injury. Learn Mem 2000;7:442-6.
18. Tomaiuolo F, Carlesimo GA, Di Paola M, et al. Gross morphology and morphometric sequelae in the hippocampus, fornix, and corpus callosum of patients with severe non-missile traumatic brain injury without macroscopically detectable lesions: a T1 weighted MRI study. J Neurol Neurosurg Psychiatry 2004;75:1314-22.
19. Tsai SY, Gildengers AG, Hsu JL, Chung KH, Chen PH, Huang YJ. Inflammation associated with volume reduction in the gray matter and hippocampus of older patients with bipolar disorder. J Affect Disord 2019;244:60-6.
20. Aziz N. Measurement of circulating cytokines and immune-activation markers by multiplex technology in the clinical setting: what are we really measuring? For Immunopathol Dis Therap 2015;6:19-22.
21. Koelman L, Pivovarova-Ramich O, Pfeiffer AFH, Grune T, Aleksandrova K. Cytokines for evaluation of chronic inflammatory status in ageing research: reliability and phenotypic characterisation. Immun Ageing 2019;16:11.
22. Belzeaux R, Lefebvre MN, Lazzari A, et al. How to: measuring blood cytokines in biological psychiatry using commercially available multiplex immunoassays. Psychoneuroendocrinology 2017;75:72-82.
23. McKay HS, Margolick JB, Martínez-Maza O, et al. Multiplex assay reliability and long-term intra-individual variation of serologic inflammatory biomarkers. Cytokine 2017;90:185-92.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Zhang LH, Yang HY, Wu J, Wu Y, Wang LL, Tong HY, Zhang J, Wang WC, Huang RY, Xu JL, Su J, Luo XR, Yin Y, Wu SH, Hu XT. Chronic intracerebroventricular administration is a reliable method in brain studies on monkeys. Ageing Neur Dis 2022;2:5. http://dx.doi.org/10.20517/and.2022.02
AMA Style
Zhang LH, Yang HY, Wu J, Wu Y, Wang LL, Tong HY, Zhang J, Wang WC, Huang RY, Xu JL, Su J, Luo XR, Yin Y, Wu SH, Hu XT. Chronic intracerebroventricular administration is a reliable method in brain studies on monkeys. Ageing and Neurodegenerative Diseases. 2022; 2(2): 5. http://dx.doi.org/10.20517/and.2022.02
Chicago/Turabian Style
Zhang, Lin-Heng, Hong-Yi Yang, Jing Wu, Yun Wu, Lu-Lu Wang, Hai-Yang Tong, Jin Zhang, Wen-Chao Wang, Rong-Yao Huang, Jiang-Lei Xu, Jing Su, Xun-Ran Luo, Yong Yin, Shi-Hao Wu, Xin-Tian Hu. 2022. "Chronic intracerebroventricular administration is a reliable method in brain studies on monkeys" Ageing and Neurodegenerative Diseases. 2, no.2: 5. http://dx.doi.org/10.20517/and.2022.02
ACS Style
Zhang, L.H.; Yang H.Y.; Wu J.; Wu Y.; Wang L.L.; Tong H.Y.; Zhang J.; Wang W.C.; Huang R.Y.; Xu J.L.; Su J.; Luo X.R.; Yin Y.; Wu S.H.; Hu X.T. Chronic intracerebroventricular administration is a reliable method in brain studies on monkeys. Ageing. Neur. Dis. 2022, 2, 5. http://dx.doi.org/10.20517/and.2022.02
About This Article
Copyright
Data & Comments
Data

 Cite This Article 15 clicks
Cite This Article 15 clicks












Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.