Recent developments in understanding brain aging: sex differences, mechanisms, and implications in diseases
Abstract
Exemplified by the disproportionate cases of Alzheimer’s disease among women, many diseases show considerable sexual disparity in the aging process. Given that such a sex bias varies significantly in different neurological conditions, considering sex differences is necessary for the diagnosis as well as the treatment of neurological disorders. However, currently, relatively few studies have specifically focused on sex differences in brain aging or the general aging process, which has prevented the development of precision medicine for these sex-different diseases. Here, we summarize age-related disparities relating to cognitive function and dysfunction for males and females from human cross-sectional and longitudinal studies. By discussing potential anatomical and physiological bases underlying such differences, we highlight the importance of sex for aging studies in this review, which may hopefully shed light on understanding the precise causes of different brain diseases.
Keywords
INTRODUCTION
According to figures from the World Health Organization (WHO), the number and proportion of people aged 60 and older are expanding and estimated to increase to 1.4 billion by 2030 and 2.1 billion by 2050, up from 1 billion in 2020[1]. The process of aging is associated with both normal and pathologic cognitive changes, which significantly affect older adults’ daily life and society. The most recent data suggest that the prevalence of dementia will double in Europe and triple worldwide by 2050. Economic costs for dementia reached 957.56 billion dollars and are set to increase to 2.54 trillion dollars worldwide[2]. Alzheimer’s disease (AD) is a main cause of dementia, the related total costs of which will reach 507.49 billion dollars by 2030 in China and 1.89 trillion by 2050[2]. Despite this, the clinical diagnosis of AD still faces many problems. There is a clear lack of precise gold standards for both diagnosis and treatment, and scientists have yet to develop multiple effective therapies for AD, especially for patients suffering in the later stages of the disease. Hence, it is clear that, for AD and other broader age-related conditions, research on aging and age-related diseases requires urgent attention.
During the human aging process, females show longer lifespans overall[3,4] but often also display more frailty than males[5]. For example, women aged 45-79 had a higher frailty index based on standards[6] including 28 variables on function, cognition, co-morbidity, health attitudes and practices, and physical performance measures[7]. This is known as the “male-female health-survival paradox”[8], and the sex variable can make a difference for health risks in males and females. To date, sex factors have attracted wide attention in the studies of human aging. In the discoveries of brain aging, sex bias has been well-recognized in the prevalence of certain brain aging-related diseases. For example, females with AD or other dementias exhibit a two-fold incidence compared with males[9]. Conversely, the prevalence of another progressive and age-related neurodegenerative disorder, Parkinson’s disease (PD), is 1.5 times more common in men than women[10]. In addition, sex and gender can affect the risk factors and disease progression of aging-related diseases such as AD[11]. Thus, it is important to understand the sex difference in changes of normal brain aging, which should provide specific clues for understanding the sex-related mechanisms for age-related diseases and, in turn, may facilitate improved and personalized care during aging.
Here, we focus on reviewing the current literature reporting the sex difference in the functional changes (cognitive decline and vulnerability to neurodegenerative diseases), structural changes, and cellular hallmark changes of normal brain aging. To address this, we used the terms “sex difference”, “brain aging”, “cognitive aging”, “brain structure”, and keywords for cellular hallmarks of brain aging (mitochondria, oxidative stress, glia, ubiquitin-proteasome system, autophagy, DNA repair, stem cell exhaustion, and aberrant neuronal network activity) to search the literature in databases such as PubMed.
We first briefly review human data relating to the sex difference in cognitive decline and vulnerability to neurodegenerative diseases in the process of normal brain aging. Next, we discuss sex difference in potential anatomical changes underlying functional changes of brain aging with human evidence. Finally, we summarize the discoveries on sex difference in cellular hallmarks such as oxidative stress of brain aging from animal experiments and human data, which may offer clues for better therapeutics to cognitive decline in aging and neurodegenerative diseases.
SEXUAL DIFFERENCES IN VULNERABILITY OF MALES AND FEMALES TO COGNITIVE DECLINE IN AGING AND IN RELATED DISEASES
Sex difference of cognitive decline in normal brain aging
Cognitive function includes a variety of mental processes such as perception, attention, memory, decision making, and language comprehension. General sex differences in specific cognitive functions have been reported, with the most accepted findings being that men outperform women on spatial-based aspects, especially visual-spatial working memory tasks[12], while females excel in verbal memory and location memory tasks[13,14]. These differences seem to remain consistent from adolescents and young adults into older ages[15,16]. In line with previous reports, several studies have demonstrated that certain cognitive functions decline along with the normal process of the aging of the human brain[17,18]. Such studies monitor the trajectories of cognition change along with the process of aging in order to pick up any related changes of cognitive function that occur concurrently with stages of the aging process.
Longitudinal studies have been demonstrated as particularly useful and applicable in the study of both the difference of cognitive performance levels and the rates of cognitive change over time. In line with the results of the cross-sectional studies mentioned above, De Frias et al.[19] found that women performed better on episodic memory tasks and men had higher visuospatial ability, and this sex difference was stable across age groups (35-80 years) over a 10-year period. When detecting the cognition decline rate between females and males with aging, although a review published in 2013 which screened 13 longitudinal studies concluded that no sex differences were found in the rate of overall cognitive decline between the ages of 60-80 years[20], there are many other investigations that have shown sex differences existed in some specific cognitive tasks or in much older age (> 80) [Table 1]. Finkel et al.[21], for example, found men had a faster linear decline than women on a card rotation test from middle age (50). Another study conducted by Casaletto et al.[22] detected the age-related cognitive decline of 314 normal adults (average 69.3) and found that men tended to develop a declining episodic memory trajectory. Meanwhile, in recent longitudinal studies, McCarrey et al.[23] administered a series of memory and other cognitive tests to participants from the Baltimore Longitudinal Study of Aging to detect the cognitive change of females and males with age. They found men showed steeper rates of decline on measures of mental status, perceptuomotor speed and integration, and visuospatial ability, but no significantly differing declines on other cognitive abilities tested compared to women[23]. However, when analyzing increased numbers of people of the older age brackets, and after adjusting for age, education, and vascular factors, one study demonstrated that women showed a steeper decline of cognition than men after 80 years old[24]. Finkel et al.[21]’s study also demonstrated that women had a faster decline in information tests than men at ages beyond 65, with a much steeper decline after 80. However, McDowell et al.[25] showed a trend for a steeper decline in men when compared with women after 80 years. Because of the complexity of human studies, it is still difficult to have a consistent conclusion, and further studies are still warranted. Nevertheless, these findings seem to indicate that women tend to show more cognitive decline at later old age than men (> 80), particularly for some for specific cognition functions, while men may have a faster cognitive decline in earlier old age (50-65).
Longitudinal studies for sex difference in normal cognitive decline
| Ref. | Age (year) | Type of cognitive task | Decline rate |
| Finkel et al.[21] | > 65 | Information test | F > M |
| 50-65 | Card rotation test | M > F | |
| Casaletto et al.[22] | 47-99 | The California verbal learning test | M > F |
| McCarrey et al.[23] | 64.9-69.7 | MMSE, fluent language production, digital symbol, card rotation | M > F |
| Proust-lima et al.[24] | > 80 | Digit symbol substitution task | F > M |
| McDowell et al.[25] | > 65 | Modified mini-mental state (3MS) cognitive Screening test | F > M (institution) |
| > 80 | Modified mini-mental state (3MS) cognitive Screening test | M > F |
Limitations and difficulties of human research techniques may contribute to these disparities. Firstly, the backgrounds of individuals may represent differences in aspects such as education[24,26], lifestyle[27], physical activities[28], and weight[29], and these factors not only affect the baseline of individual cognitive function but also affect the rate of cognitive decline. This makes the study on the effect of the single factor of sex on the rate of cognitive decline difficult to isolate. Moreover, in longitudinal studies, subjects are incorporated into such studies from many different age groups, in which case a limited number of subjects will be of the same age, despite a large sample of total subjects being involved. Different cohorts also show different aspects of cognitive aging[30,31]. All these factors may cause discrepancies in results. To distinguish the true effect of sex on cognitive decline with aging, larger sample sizes of confirmed similar backgrounds and the same ages should be involved, and the observation durations should be extended for these groups.
Sex differences in vulnerability to aging associated cognitive disorders
Brain aging is a natural process that results in a certain level of associated cognitive decline. However, as the brain ages, it is more susceptible to neurodegenerative diseases such as Alzheimer’s disease (AD), Lewy body dementia (LBD), frontotemporal dementia (FTD), or Parkinson’s disease (PD). These diseases usually occur in later life, worsening with subsequent aging. They often manifest with increasing age-related cognitive impairment, finally leading to dementia. Unlike the inconsistent results for the sex difference of cognition decline in normal brain aging, relatively consistent results have been demonstrated relating to females and males for some aspects of the vulnerabilities to these diseases [Table 2].
Sex difference of neurodegenerative diseases
| Neurodegenerative diseases | Factors | Sex difference | Ref. |
| Alzheimer’s disease (AD) The leading cause of dementia, which accounts for 60%-80% of dementia cases | Number | F > M | Chêne et al.[32] Hebert et al.[33] Alzheimer’s Association 2021[34] |
| Prevalence | F > M | Plassman et al.[35] Prince et al.[36] Prince et al.[37] | |
| No difference | Prince et al.[36] Rocca[152] | ||
| Incidence | F > M | Fitzpatrick et al.[40] Prince et al.[41] Ruitenberg et al.[42] Rizzi et al.[43] Chen et al.[44] Yamada et al.[45] | |
| F = M (equal) | Tom et al.[38] Zahodne et al.[39] | ||
| Cognitive decline rate | F > M | Hebert et al.[33] Holland et al.[46] Tifratene et al.[47] Lin et al.[48] Laws et al.[49] Gamberger et al.[50] | |
| Lewy body dementia (LBD) The second most prevalent cause of neurodegenerative dementia | Number | M > F | Nelson et al.[54] |
| Incidence | M > F | Savica et al.[55] Goodman et al.[56] | |
| Frontotemporal dementia (FTD) The third most prevalent form of neurodegenerative dementias | Prevalence | M > F | Goodman et al.[56] Mercy et al.[58] |
| F > M | Bernardi et al.[59] Ikeda et al.[60] | ||
| No difference | Borroni et al.[61] | ||
| Parkinson’s disease (PD) A movement disorder that can also lead to dementia | Prevalence | M > F | Pringsheim et al.[63] (worldwide) Hirsch et al.[64] Abbas et al.[65] GBD 2016 Neurology Collaborators[66] |
| No difference | Pringsheim et al.[63] (Asia) Taylor et al.[67] | ||
| Cognitive decline | M > F | Reekes et al.[71] |
AD is the leading cause of dementia, which accounts for 60%-80% of dementia cases. Over the past 20 years, many studies have investigated sex differences in the risk, incidence, prevalence, or development of AD. For the analysis of risk, the Framingham Heart Study showed that, for a 65-year-old woman, the risk of AD over her remaining lifetime was 21.2%, while for a man, it was 11.6%. Correspondingly, the ratio of female to male risk for AD was noted as about 2:1[32]. For overall numbers, many epidemiological studies of varied global locations also highlighted a higher number of women than men with AD[33,34]. The prevailing explanation for such a difference is that women live longer than men on average, and that the incidences of AD correspond with increased age[32]. The argument could therefore be formulated that any apparent sex-based difference for this disease is simply due to the increased average longevity for women. However, upon a more specific analysis of prevalence, results seem to conflict with the lifespan explanation.
LBD is a neurodegenerative disease with abnormal α-synuclein accumulation (Lewy body proteins) in neurons, which can cause cognitive decline. LBD is the second most frequent form of neurodegenerative dementia. Reviews show the prevalence of LBD ranges 0.3%-24% in the general population and 3%-7% in the patients with dementia[52,53]. In accessing the registry autopsy series from the University of Kentucky Alzheimer’s Disease Center and the National Alzheimer’s Coordinating Center, researchers found that the number of male patients dying with Lewy body-associated pathologies was three times that of females[54]. Similarly, a study on the population of Olmsted County, Minnesota, showed that men had a higher incidence of LBD than women across the age spectrum[55]. A study on the prevalence of dementia subtypes in United States Medicare showed the same result[56]. FTD is considered the third most frequent form of neurodegenerative dementia with more relatively young patients than other types of dementia (< 65)[57]. While many studies have demonstrated sex differences in the prevalence of this disorder, no clear consensus has been reached. Studies in the population of Cambridgeshire, UK, and a United States Medicare study both showed greater FTD prevalence in men than women[56,58]. However, many other studies failed to support these results[59-61]. The discrepancy here may be due to difficulties in the exact diagnosis of FTD, which presents with similar clinical symptoms to late onset psychiatric disorders and amyotrophic lateral sclerosis (ALS). Recently, Curtis’s meta-analysis focusing on the sex difference of the prevalence of genetic mutations in FTD and ALS indicated a higher prevalence of progranulin (GRN)-muted FTD in female patients but no sex differences in chromosome 9 open reading frame 72 (C9orf72)- and microtubule-associated protein tau related FTD (MFTD), which should further help clarify the sex differences of prevalence of FTD[62]. Another neurogenerative disease is PD. PD is a movement disorder with bradykinesia, rigidity, tremor at rest, gait disturbance, and difficulty with speech. PD can also lead to dementia, and the proportion of patients with PD who are also diagnosed with Parkinson’s disease dementia ranges from 10% to 15%. Studies are fairly consistent in demonstrating that there is a higher prevalence of PD presented among men than women from worldwide epidemiological data[63-66], especially in Western and South American populations[10,67-69]. However, there are reports showing the prevalence rates were almost equal between men and women in Asian populations[63,67]. Thus, there appears to be a difference between Asian and Western populations, which may stem from sex different behaviors such as smoking, methodologies, genetics, and ethnicity[65,70]. For the cognitive decline in PD, Reekes et al.[71] indicated that males with PD have significantly greater executive and processing speed impairments compared to women.
As mentioned above, the aging brain undergoes cognitive functional change and becomes increasingly susceptible to a number of cognitive diseases. Although no results have consistently or conclusively shown differences in cognitive decline rate between females and males, apparent sex differences have been shown to be involved in the cognitive performance and disease susceptibility of the elderly. In addition, females have been demonstrated as more susceptible to AD, and males are more vulnerable to LBD and PD. The underlying mechanism for such sex-based differences in brain aging behaviors and related diseases is a key area for further study.
BRAIN STRUCTURE AS A BASIS FOR SEX DIFFERENCES IN BRAIN AGING
To explain how function changes with aging, the most widely investigated aspect is the structural changes of the aging brain. With the advantages of noninvasive imaging techniques, researchers were able to study the aging brain in healthy living individuals. As many healthy volunteers were incorporated into such studies, this provided the opportunity to analyze the sex differences of the human brain anatomy relating to aging. Using magnetic resonance imaging, many researchers found more profound age-related decline in cortical grey matter volume in males than females[72-74]. However, there are many investigations that do not support the hypothesis that the effect of aging is accelerated in men and have failed to find age-by-sex interactions in adult and elderly populations[75-77]. When considering the sex differences of subcortical gray matter structure in the aging brain, conclusions are no more consistent [Table 3]. The subcortical structures studied include the basal ganglia (caudate, nucleus accumbens, putamen, and pallidum), thalamus, hippocampus, and amygdala. Among these, the hippocampus is the most studied, and some hippocampus studies have reported that females have larger volumes in the aging brain[78-80] while others have opposing results[81]. In old age, for thalamus, some studies found the male had a larger volume[72,80], while others opposed this[82]. When correcting for brain volume, Li et al.[78] found no significant sex difference in the relative volume of thalamus. Similarly, for the caudate, some found females had larger volume[83,84], while others found males had larger volume[78,80]. More consistently, the amygdala[78,85], pallidum[78,80], and putamen[78,80] have been invariably found to be larger in males.
Sex difference of subcortical regions in the aging brain of cross-sectional studies
| Subcortical regions | Ref. | Age (year) | Sex difference of volume in older age (> 45) | Sex difference of decline rate in older age (> 45) |
| Hippocampus | Li et al.[78] | 19-70 | F > M (relative volume) | M > F (relative volume) |
| Nemeth et al.[79] | 21-58 | F > M | M > F | |
| Wang et al.[80] | 19-86 | F > M (> 70) | M > F | |
| Goto et al.[81] | 41-77 | M > F (absolute volume) | F > M (absolute volume) | |
| Thalamus | Sullivan et al.[72] | 20-85 | M > F | Similar |
| Li et al.[78] | 19-70 | Similar (relative volume) | Similar (relative volume) | |
| Wang et al.[80] | 19-86 | M > F | M > F | |
| Takahashi et al.[82] | 20 to ≥ 80 | F > M | M > F | |
| Caudate nuclei | Li et al.[78] | 19-70 | M > F (relative volume) | F > M (relative volume) |
| Wang et al.[80] | 19-86 | M > F | Similar | |
| Good et al.[83] | 18-79 | F > M | No test | |
| Luders et al.[84] | 18-82 | F > M | No test | |
| Putamen | Li et al.[78] | 19-70 | M > F (relative volume) | F > M (relative volume) |
| Wang et al.[80] | 19-86 | M > F | M > F (right) | |
| Pallium | Li et al.[78] | 19-70 | M > F (relative volume) | F > M (relative volume) |
| Wang et al.[80] | 19-86 | M > F | M > F (right) | |
| Accumbens | Wang et al.[80] | 19-86 | Similar | Similar |
| Amygdala | Li et al.[78] | 19-70 | M > F (relative volume) | F > M (relative volume) |
| Cheng et al.[85] | 20-50 | M > F | No test |
Differences in these findings may be due to differences in the age range of subjects evaluated and methods used for analysis. The previous findings of brain structure changes in the aged brain of females and males are mainly based on cross-sectional studies, which only show the status at one specific time or with different status at different specific times. Longitudinal studies may be better suited to address the conflicts in cross-sectional studies. Over the last 15 years, increasing numbers of longitudinal studies have been performed to investigate the rate of brain change with aging. Among them, some studies have paid attention to the sex difference in aging[86-90]. Taki et al.[86]’s studies showed the annual percentage change in the grey matter ratio (APCGMR) in the older female group was substantially lower than in the older male group, using such a longitudinal design running over a period of over six years in 381 healthy community-dwelling individuals. Jiang et al.[88] chose individuals aged 70-90 years as subjects. After a two-year follow-up, they found that women had thicker cortical regions but greater rates of cortical atrophy[88]. For the structural changes of cortex subregions, Pfefferbaum et al.[87] focused on the change of regional brain volume with aging in longitudinal studies. They found a more rapid increase of lateral ventricle volume and Sylvian fissures and more rapid decline of the centrum semiovale, anterior cingulate, parietal and precentral cortices, and thalamus in older men than older women, especially in those beyond 60 years of age[87]. Narvacan et al.[89] scanned a cohort of 55 subjects approximately three years apart. While finding that overall males had larger volumes than females for all subcortical structures, no sex differences in trajectories of change were detected[89]. Such differences may stem from longitudinal studies which can be limited by the age range of participants, sex distribution of the samples, or scanning intervals. In a recent study, Vinke et al.[90] used the Rotterdam study[91] to understand the different aspects in brain aging of middle- and old-aged males and females based on a large prospective population-based cohort study. Their analysis showed that an earlier acceleration of decrease for normal-appearing white matter volume, gray matter volume, total brain volume, hippocampus volume, and pallidum volume and increased cerebrospinal fluid volume had occurred in men compared with women. Meanwhile, men tended to have a higher prevalence of focal lesions (microbleeds, lacunes, and cortical infarcts) compared with women. Although shorter time intervals and less time for scanning posed some limitations for the reliable representation of the longitudinal effect of those of older ages, this study, with the largest sample used for aging-related research, provides good background information for understanding the different changes in the female and male brain due to aging.
As for other studies dealing with functional age-related change in the human brain, limitations exist in both cross-sectional and longitudinal studies for investigating how the brain’s structure changes with aging. Although no consistent results have been reached for such sex differences in brain structural changes, most studies have indicated that males have accelerated atrophy in the grey matter of the cortex. This may support some findings for the faster cognitive decline in males mentioned above. The noted differences in changes for different subcortical regions may help us to understand why females outperformed males in some specific tasks while males outperformed females in others. In their review, Nemeth et al.[79] demonstrated the possible functional consequences of sex difference of the subcortical grey matter, noting that their findings were relevant for dementia occurrence. However, the direct link among brain structures, cognition, and behavior is not currently clear and requires further investigation.
CELLULAR BASIS FOR SEX DIFFERENCES IN BRAIN AGING
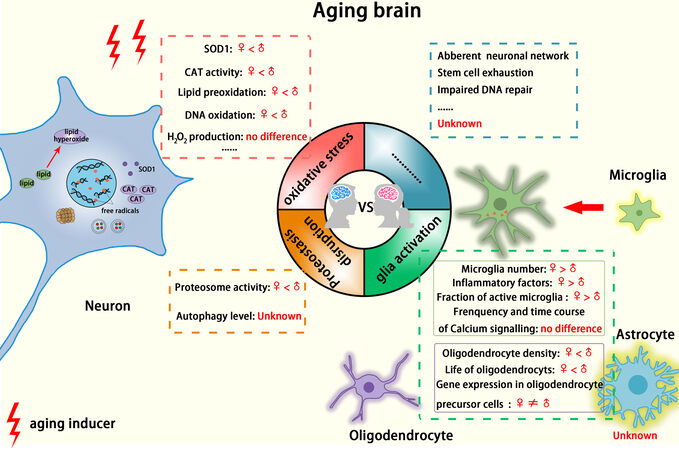
Mattson and Arumugam[92] 2018 paper summarizes these findings well and organizes the main aspects of brain aging into nine hallmarks: (1) mitochondrial dysfunction; (2) oxidative damage; (3) impaired cellular “waste disposal” mechanisms (autophagy-lysosome and proteasome functionality); (4) impaired adaptive stress response signaling; (5) impaired DNA repair; (6) aberrant neuronal network activity; (7) dysregulated neuronal Ca2+ handling; (8) stem cell exhaustion; and (9) glia cell activation and inflammation. To date, several studies (mainly from animal experiments) have shown sex differences for these hallmarks, indicating possible cellular and molecular mechanisms for sex disparity of brain aging [Figure 1].
Figure 1. Sex differences implicated in cellular hallmarks of the aging brain. Shown in colored sections and dashed boxes, sex differences may exist in some hallmarks of brain aging.
Sex difference in mitochondrial dysfunction and oxidative stress with brain aging
The mitochondrion is an important organelle in the cell, which plays crucial roles in ATP production, storage calcium ions, and the regulation of cellular proliferation[93]. Sex difference has been found in many aspects of brain mitochondrial function including morphology, pathways of biogenesis, autophagy, cell death, calcium, and redox homeostasis[94]. However, such results have been mainly based on investigations of the adult brain or injured brain. Investigations on sex differences of brain-based mitochondrial dysfunction related to the normal aging process are relatively sparse. However, sex factors of redox homeostasis have been directly studied relating to brain aging, where the mechanism of the balance of free radical production and antioxidants is required to maintain redox homeostasis.
Upon aging, brain neurons tend to suffer from oxidative damage by excessive generation of free radicals and reduced antioxidant defense. Animal studies showed that, in young adult rats, females exhibit lower release of cytochrome c and lower levels of mitochondrial hydrogen peroxide than males[95]. Moreover, in rats of similar ages, female brain mitochondria generated half the amount of peroxides and imposed dramatically less oxidative damage to mitochondrial DNA than those of males[96]. In contrast, Guevara et al.[97,98]’s studies on rat brains of different ages (6, 12, 18, and 24 months old) showed no significant sex-based differences for H2O2 production in any age class, despite H2O2 production being increased with age in both sexes.
For the antioxidant system, studies on rodents showed that young female brains have higher expression or activities of the antioxidant enzymes SOD and CAT[99-101]. However, upon brain aging, the factors of sex difference relating to oxidative stress become complicated. Although higher antioxidant defense occurred in young female rats, after ovariectomy, mitochondrial peroxide and glutathione (GSH) levels in females became similar to those of males[96], indicating decreased antioxidant ability in menopausal female rats. Accordingly, other non-human studies also showed higher CAT activity in the aging (18-20 months) male mouse brain and higher SOD1 protein levels in the brain of aged (28-month-old) males[102]. Similar results were found in Human studies. Viña and Borrás[103] proposed that young females have a better ability to fight against oxidative stress with higher antioxidant levels prior to menopause. Mandal et al.[104] also found that young females (± 26 years old) have higher GSH levels than young men in the frontal and parietal cortex. Interestingly, the GSH levels decrease in the brains of older women (56 years old) compared with the younger women[104], indicating that the antioxidant ability changes with aging in women. In line with this, Rekkas et al.[105] found that oxidative stress in the brain increased rapidly in perimenopausal women. Bilateral oophorectomy-induced menopause was associated with increases in the GSH/GSSG ratio (increase in GSH, decrease in GSSG) and reduction in SOD and glutathione peroxidase (Gpx) mRNA expression[106], suggesting that women had decreased antioxidant ability after menopause.
When detecting oxidative damage in the brain, several studies in rodents have demonstrated males may exhibit greater oxidative damage, expressed as higher DNA oxidation[107] or higher levels of lipid peroxidation[107-109], than age-matched females over relatively young ages. This may be caused by lower free radical production and higher antioxidant defense in young females. However, other studies show no such sex differences[110,111] or higher oxidative damage in females[100,112]. These differences may stem from the different brain regions under examination or the different times of detection. Guevara et al.[97,98]’s studies on rats of different ages (6, 12, 18, and 24 months old) showed lesser oxidative damaged proteins and lipids in female brains, even in old age, an observation which they attributed in part to higher Gpx activity.
Overall, these findings on the sex difference in oxidative stress in the aging brain suggest that young females have a better ability to defend against oxidative stress, but these advantages may be lost upon aging, especially beyond menopause. As reviewed in Grimm et al.[115]’s paper, the change of redox state with aging is associated with variation in sex steroid levels. This finding may aid in the understanding of sex differences in vulnerability to neurodegenerative disease[115].
Sex differences in glia cell activation upon brain aging
Glial cells are distinguished from neurons in the brain and play important roles in brain function via facilitating crosstalk with neurons, maintaining the normal function of neurons, and defending against harmful stimuli. There are different types of glial cells in the brain, including astrocytes, microglia, and oligodendrocytes, each with distinct functions. Oligodendrocytes insulate axons and provide them with trophic support[116]; microglia are regarded as macrophages participating in local immunity[117]; and astrocytes provide biochemical support of neuronal activities by facilitating the appropriate glial surroundings in conjunction with microglia[117,118]. Various wide-ranging studies have demonstrated sex differences related to glial cells in physiological conditions or in response to pathological insults[119-121]. However, sex differences in aged glial cells remain relatively under-studied, and the discoveries mainly stem from animal experiments.
Sex differences in microglia are the most studied sex-related aspect in the process of brain aging, with previous studies quantifying changes of microglia in the aging brain[122,123]. Mouton et al.[123]’s study showed that, in both young and old mice, females had more astrocytes and microglia in DG and CA1 of the hippocampus than did age-matched male mice. In addition, with the development of sequencing technology, recent studies have paid increasing attention to the gene expression relationships for microglia in aging[124,125]. Mangold et al.[126] also detected the sex differences for microglial gene expression in the mouse hippocampus and cortex. They found that inflammatory genes were more highly expressed in microglia of older females than in corresponding older males[126]. Importantly, using single-cell RNA-sequencing analysis, Sala Frigerio et al.[127] found that aging or progressive amyloid-β accumulation accelerated the two main activated microglia states and that female mice progressed faster in this than males, which also converged with the pathway of sex differences relating to aging and AD. In addition, Kang et al.[128] mentioned in their article that tauopathy, amyloidosis, and aging had been shown to share a common APOE-driven transcriptional signature in microglia, which indicates that the increased expression of many of these transcripts of microglia in older mouse brains may be related to increased susceptibility to Alzheimer’s disease in females. As regards to the functions of microglia in aging, one recent study on phagocytosis showed that aged female microglia had a greater ability for phagocytosis of neuronal debris, but they had lost their ability to adapt their phagocytic activity to inflammatory conditions[129]. Another study on microglial function, analyzing microglial Ca2+ signaling and process motility, suggested “faster aging” for microglia in female mice[130]. Taken together, the more active/faster aging microglia in older females may render them more vulnerable to some age-related neurodegenerative disease such as AD.
Relatively few studies have focused on astrocytes and oligodendrocytes in aging. Research on astrocytes has mainly been conducted in vitro via the detection of differences in the changes between the sexes under specific stimuli or in response to various pathological insults[131]. No specific analysis of the sex differences relating to the aging process seems to have been conducted relating to astrocytes. For oligodendrocytes, Cerghet et al.[132,133] examined the sex difference of oligodendrocytes in the rat and mouse brain and found that the density of oligodendrocytes in the corpus callosum, fornix, and myelin proteins and myelin gene expression were all greater in males, and shorter life of oligodendrocytes was noted in females, a finding which did not simply represent younger mice and rats, but also held true for old mice. These differences for oligodendrocytes and myelin may be associated with the sex difference in the white matter volume in the adult and aging brain, which is discussed in “BRAIN STRUCTURE AS A BASIS FOR SEX DIFFERENCES IN BRAIN AGING”. Oligodendrocyte precursor cells can generate new mature oligodendrocytes to defend against myelin impairment in the adult brain. Transcriptomic analyses have identified sex differences in oligodendrocyte precursor cells in the expression of genes encoding for proteins involved in the cell cycle, proliferation, maturation, and myelination, among other functions[134,135]. This difference renders older (12-month-old) female rats with greater abilities of remyelination than males after demyelination lesions[136].
Sex differences in proteasome degradation and autophagy upon brain aging
In most organisms, a balance in the protein system, which forms the basis for gene expression and protein synthesis and degradation, is important for the normal function of cells. Aging often shifts this balance with the subsequently altered gene expressions and protein synthesis and disrupted protein degradation, resulting in some notable pathological features[137]. In recent studies, many molecules have been demonstrated to be associated with brain aging including SFRS11[138], CD22[139], REST[140], and BAZ-2 and SET-6[141], the expressions of which change along with the aging process. However, no particular sex differences have been noted for these. Whether this is due to there not being any notable sex differences, or that this is an aspect yet to be properly examined, remains to be clarified.
Another important part of the protein system is protein degradation, which has been verified to be disrupted with brain aging[142]. In cells, two main protein degradation systems exist, namely the ubiquitin-proteasome system (UPS) and autophagy[143,144]. A few non-human data demonstrate that sex differences exist in these protein degradation systems of the aged brain. Ding and Keller[145]’s study showed that proteasome inhibition occurs with aging in the central nervous system, while Zeng et al.[146]’s study demonstrated that, in older (15-month-old) female mice and rats, catalytic activities of the proteasome are decreased in the cortex, striatum, cerebellum, globus pallidus, and substantia nigra with aging. In
As for autophagy, while no direct articles have reported sex differences in the normal aging brain, multiple studies have demonstrated its role in AD[150]. Many investigations on other tissues (not brain tissue) have shown that females appear to have overall lower levels of autophagy, and ovariectomized animals show increased basal levels of autophagy in several cell types[151]. This highlights the changes in the nature and extent of autophagy after menopause in women. Although more research is needed, considering the results of Jenkin et al.[147]’s study, younger females show an overall higher proteostasis capacity, where there may be a particularly well-established balance in the two types of protein degradation systems, but these could then become disrupted upon aging, rendering elderly women more vulnerable to a number of associated diseases.
For the many other hallmarks of brain aging, few studies have paid attention to any potential sex differences. Although many of the above-noted differences in the cellular changes between the female and male aging brains are not fully understood, these discoveries will likely attract increasing numbers of researchers to consider the sex factor and to further illuminate the related cellular mechanisms. These will help link the hormonal effects with those that relate to cognition and behavior, in the process of aging.
CONCLUSION
Sex differences in the brain, as they function in developmental and adult stages, have been widely investigated. However, studies on the sex factors related to the aging brain are lagging behind and deserve increasing attention, particularly under the current situation of a rapidly aging global population. Although there are no consistent general conclusions related to sex differences on cognitive decline with aging, the differences of some aspects of cognitive performance in older adults and the increased vulnerability of females and males to various aspects of dementia are becoming fairly well established, especially for AD and PD where sex is regarded as a primary risk factor for these neurodegenerative diseases. Specifically, many studies have demonstrated that women have a higher prevalence of AD than age-matched men and exhibit faster cognitive decline beyond AD diagnosis. Conversely, almost all recent studies have demonstrated that men have a higher prevalence of PD. However, for treatment, the current interventions for dementia often fail to consider the sex factor. One possible reason may be that the underlying mechanisms of sex difference for the functional changes that occur during the process of brain aging are not yet fully clear and that present techniques and other socioeconomic factors render such factors difficult to examine in detail. However, it is clear that age-related cohorts need to be established and traced to provide information about how human aging-related phenotypes and molecular changes upon aging relate to sex, in order to guide future health improvements. Beyond human-based studies, to unpack the possible structural and cellular mechanisms for sex differences of the aging brain, the use of animal models should be increasingly established, with experiments designed that can incorporate the benefits of the model animal’s simple genetic background. With a better understanding of the biological mechanism for the sex differences in brain aging, we can better understand the overall functional changes in the brain, elucidate how sex creates differences in disease risk, lay a stronger foundation for dealing with the newly emerging aspects of neurodegenerative disease, explore more directly the biomarkers for brain aging, and further promote personalized medicine that incorporates the factor of sex for improved and more individualized disease treatment.
DECLARATIONS
Authors’ contributionsMade contributions to conception of this review article: Yang J, Ma H
Screened and gathered articles and wrote the abstract and introduction: Qu J
Wrote the other sections and made tables: Yang J
Availability of data and materialsNot applicable.
Financial support and sponsorshipThis study was supported by the National Key R&D Program of China to Ma H (grant number 2019YFA0508603); Science and Technology Innovation 2030-Major Project to Ma H (2021ZD0203501); the National Natural Science Foundation of China (grant numbers 81930030, 31771109, and 31722023 to Ma H; 81901154 to Yang J); Project for Hangzhou Medical Disciplines of Excellence; Key Project for Hangzhou Medical Disciplines.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
REFERENCES
1. Aging and health. Available from: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health [Last accessed on 25 Mar 2022].
2. Jia J, Wei C, Chen S, et al. The cost of Alzheimer’s disease in China and re-estimation of costs worldwide. Alzheimers Dement 2018;14:483-91.
3. Sampathkumar NK, Bravo JI, Chen Y, et al. Widespread sex dimorphism in aging and age-related diseases. Hum Genet 2020;139:333-56.
4. Bronikowski AM, Altmann J, Brockman DK, et al. Aging in the natural world: comparative data reveal similar mortality patterns across primates. Science 2011;331:1325-8.
5. Fan J, Yu C, Guo Y, et al. Frailty index and all-cause and cause-specific mortality in Chinese adults: a prospective cohort study. Lancet Public Health 2020;5:e650-60.
6. Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr 2008;8:24.
7. Gordon EH, Peel NM, Samanta M, Theou O, Howlett SE, Hubbard RE. Sex differences in frailty: a systematic review and meta-analysis. Exp Gerontol 2017;89:30-40.
8. Oksuzyan A, Petersen I, Stovring H, Bingley P, Vaupel JW, Christensen K. The male-female health-survival paradox: a survey and register study of the impact of sex-specific selection and information bias. Ann Epidemiol 2009;19:504-11.
9. Rajan KB, Weuve J, Barnes LL, McAninch EA, Wilson RS, Evans DA. Population estimate of people with clinical Alzheimer’s disease and mild cognitive impairment in the United States (2020-2060). Alzheimers Dement 2021;17:1966-75.
10. Moisan F, Kab S, Mohamed F, et al. Parkinson disease male-to-female ratios increase with age: French nationwide study and meta-analysis. J Neurol Neurosurg Psychiatry 2016;87:952-7.
11. Nebel RA, Aggarwal NT, Barnes LL, et al. Understanding the impact of sex and gender in Alzheimer’s disease: a call to action. Alzheimers Dement 2018;14:1171-83.
12. Voyer D, Voyer SD, Saint-Aubin J. Sex differences in visual-spatial working memory: a meta-analysis. Psychon Bull Rev 2017;24:307-34.
13. Voyer D, Postma A, Brake B, Imperato-McGinley J. Gender differences in object location memory: a meta-analysis. Psychon Bull Rev 2007;14:23-38.
14. Asperholm M, Högman N, Rafi J, Herlitz A. What did you do yesterday? Psychol Bull 2019;145:785-821.
15. Jansen P, Kaltner S. Object-based and egocentric mental rotation performance in older adults: the importance of gender differences and motor ability. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 2014;21:296-316.
16. Munro CA, Winicki JM, Schretlen DJ, et al. Sex differences in cognition in healthy elderly individuals. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 2012;19:759-68.
17. Harada CN, Natelson Love MC, Triebel KL. Normal cognitive aging. Clin Geriatr Med 2013;29:737-52.
19. de Frias CM, Nilsson LG, Nilsson A. Sex differences in cognition are stable over a 10-year period in adulthood and old age. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 2006;13:574-87.
20. Ferreira L, Ferreira Santos-Galduróz R, Ferri CP, Fernandes Galduróz JC. Rate of cognitive decline in relation to sex after 60 years-of-age: a systematic review. Geriatr Gerontol Int 2014;14:23-31.
21. Finkel D, Reynolds CA, Berg S, Pedersen NL. Surprising lack of sex differences in normal cognitive aging in twins. Int J Aging Hum Dev 2006;62:335-57.
22. Casaletto KB, Elahi FM, Staffaroni AM, et al. Cognitive aging is not created equally: differentiating unique cognitive phenotypes in “normal” adults. Neurobiol Aging 2019;77:13-9.
23. McCarrey AC, An Y, Kitner-Triolo MH, Ferrucci L, Resnick SM. Sex differences in cognitive trajectories in clinically normal older adults. Psychol Aging 2016;31:166-75.
24. Proust-Lima C, Amieva H, Letenneur L, Orgogozo JM, Jacqmin-Gadda H, Dartigues JF. Gender and education impact on brain aging: a general cognitive factor approach. Psychol Aging 2008;23:608-20.
25. McDowell I, Xi G, Lindsay J, Tuokko H. Canadian study of health and aging: study description and patterns of early cognitive decline. Aging, Neuropsychology, and Cognition 2004;11:149-68.
26. Wilson RS, Hebert LE, Scherr PA, Barnes LL, Mendes de Leon CF, Evans DA. Educational attainment and cognitive decline in old age. Neurology 2009;72:460-5.
27. Yu F, Ryan LH, Schaie KW, Willis SL, Kolanowski A. Factors associated with cognition in adults: the Seattle Longitudinal Study. Res Nurs Health 2009;32:540-50.
28. Finkel D, Andel R, Gatz M, Pedersen NL. The role of occupational complexity in trajectories of cognitive aging before and after retirement. Psychol Aging 2009;24:563-73.
29. Noh HM, Han J, Kim YJ, Jung JH, Roh YK, Song HJ. Sex differences in the relationship between cognitive impairment and overweight or obesity in late life: a 3-year prospective study. Medicine (Baltimore) 2019;98:e14736.
30. Gerstorf D, Ram N, Hoppmann C, Willis SL, Schaie KW. Cohort differences in cognitive aging and terminal decline in the Seattle Longitudinal Study. Dev Psychol 2011;47:1026-41.
31. Karlsson P, Thorvaldsson V, Skoog I, Gudmundsson P, Johansson B. Birth cohort differences in fluid cognition in old age: comparisons of trends in levels and change trajectories over 30 years in three population-based samples. Psychol Aging 2015;30:83-94.
32. Chêne G, Beiser A, Au G, et al. Gender and incidence of dementia in the Framingham Heart Study from mid-adult life. Alzheimers Dement 2015;11:310-20.
33. Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology 2013;80:1778-83.
35. Plassman BL, Langa KM, Fisher GG, et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology 2007;29:125-32.
36. Wimo A, Ali GC, Guerchet M, Prince M, Prina M, Wu YT. World Alzheimer Report 2015 - The Global Impact of Dementia: An analysis of prevalence, incidence, cost and trends. Available from: https://www.alzint.org/resource/world-alzheimer-report-2015/ [Last accessed on 25 Mar 2022].
37. Prince M, Ali GC, Guerchet M, Prina AM, Albanese E, Wu YT. Recent global trends in the prevalence and incidence of dementia, and survival with dementia. Alzheimers Res Ther 2016;8:23.
38. Tom SE, Hubbard RA, Crane PK, et al. Characterization of dementia and Alzheimer’s disease in an older population: updated incidence and life expectancy with and without dementia. Am J Public Health 2015;105:408-13.
39. Zahodne LB, Schofield PW, Farrell MT, Stern Y, Manly JJ. Bilingualism does not alter cognitive decline or dementia risk among Spanish-speaking immigrants. Neuropsychology 2014;28:238-46.
40. Fitzpatrick AL, Kuller LH, Ives DG, et al. Incidence and prevalence of dementia in the Cardiovascular Health Study. J Am Geriatr Soc 2004;52:195-204.
41. Prince M, Acosta D, Ferri CP, et al. Dementia incidence and mortality in middle-income countries, and associations with indicators of cognitive reserve: a 10/66 Dementia Research Group population-based cohort study. Lancet 2012;380:50-8.
42. Ruitenberg A, Ott A, van Swieten JC, Hofman A, Breteler MM. Incidence of dementia: does gender make a difference? Neurobiol Aging 2001;22:575-80.
43. Rizzi L, Rosset I, Roriz-Cruz M. Global epidemiology of dementia: Alzheimer’s and vascular types. Biomed Res Int 2014;2014:908915.
44. Chen R, Hu Z, Wei L, Ma Y, Liu Z, Copeland JR. Incident dementia in a defined older Chinese population. PLoS One 2011;6:e24817.
45. Yamada M, Mimori Y, Kasagi F, et al. Incidence of dementia, Alzheimer disease, and vascular dementia in a Japanese population: Radiation Effects Research Foundation adult health study. Neuroepidemiology 2008;30:152-60.
46. Holland D, Desikan RS, Dale AM, McEvoy LK. Alzheimer’s Disease Neuroimaging Initiative. Higher rates of decline for women and apolipoprotein E epsilon4 carriers. AJNR Am J Neuroradiol 2013;34:2287-93.
47. Tifratene K, Robert P, Metelkina A, Pradier C, Dartigues JF. Progression of mild cognitive impairment to dementia due to AD in clinical settings. Neurology 2015;85:331-8.
48. Lin FC, Chuang YS, Hsieh HM, et al. Early statin use and the progression of Alzheimer disease: a total population-based case-control study. Medicine (Baltimore) 2015;94:e2143.
49. Laws KR, Irvine K, Gale TM. Sex differences in cognitive impairment in Alzheimer’s disease. World J Psychiatry 2016;6:54-65.
50. Gamberger D, Lavrač N, Srivatsa S, Tanzi RE, Doraiswamy PM. Identification of clusters of rapid and slow decliners among subjects at risk for Alzheimer’s disease. Sci Rep 2017;7:6763.
51. Todd S, Barr S, Roberts M, Passmore AP. Survival in dementia and predictors of mortality: a review. Int J Geriatr Psychiatry 2013;28:1109-24.
52. Vann Jones SA, O’Brien JT. The prevalence and incidence of dementia with Lewy bodies: a systematic review of population and clinical studies. Psychol Med 2014;44:673-83.
53. Hogan DB, Fiest KM, Roberts JI, et al. The prevalence and incidence of dementia with Lewy bodies: a systematic review. Can J Neurol Sci 2016;43 Suppl 1:S83-95.
54. Nelson PT, Schmitt FA, Jicha GA, et al. Association between male gender and cortical Lewy body pathology in large autopsy series. J Neurol 2010;257:1875-81.
55. Savica R, Grossardt BR, Bower JH, Boeve BF, Ahlskog JE, Rocca WA. Incidence of dementia with Lewy bodies and Parkinson disease dementia. JAMA Neurol 2013;70:1396-402.
56. Goodman RA, Lochner KA, Thambisetty M, Wingo TS, Posner SF, Ling SM. Prevalence of dementia subtypes in United States Medicare fee-for-service beneficiaries, 2011-2013. Alzheimers Dement 2017;13:28-37.
58. Mercy L, Hodges JR, Dawson K, Barker RA, Brayne C. Incidence of early-onset dementias in Cambridgeshire, United Kingdom. Neurology 2008;71:1496-9.
59. Bernardi L, Frangipane F, Smirne N, et al. Epidemiology and genetics of frontotemporal dementia: a door-to-door survey in southern Italy. Neurobiol Aging 2012;33:2948.e1-2948.e10.
60. Ikeda M, Ishikawa T, Tanabe H. Epidemiology of frontotemporal lobar degeneration. Dement Geriatr Cogn Disord 2004;17:265-8.
61. Borroni B, Alberici A, Grassi M, et al. Is frontotemporal lobar degeneration a rare disorder? J Alzheimers Dis 2010;19:111-6.
62. Curtis AF, Masellis M, Hsiung GR, et al. Sex differences in the prevalence of genetic mutations in FTD and ALS: a meta-analysis. Neurology 2017;89:1633-42.
63. Pringsheim T, Jette N, Frolkis A, Steeves TD. The prevalence of Parkinson’s disease: a systematic review and meta-analysis. Mov Disord 2014;29:1583-90.
64. Hirsch L, Jette N, Frolkis A, Steeves T, Pringsheim T. The incidence of Parkinson’s disease: a systematic review and meta-analysis. Neuroepidemiology 2016;46:292-300.
65. Abbas MM, Xu Z, Tan LCS. Epidemiology of Parkinson’s disease-East versus West. Mov Disord Clin Pract 2018;5:14-28.
66. GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2019;18:459-80.
67. Taylor KS, Cook JA, Counsell CE. Heterogeneity in male to female risk for Parkinson’s disease. J Neurol Neurosurg Psychiatry 2007;78:905-6.
68. de Lau LM, Verbaan D, Marinus J, van Hilten JJ. Survival in Parkinson’s disease. Relation with motor and non-motor features. Parkinsonism Relat Disord 2014;20:613-6.
69. Pinter B, Diem-Zangerl A, Wenning GK, et al. Mortality in Parkinson’s disease: a 38-year follow-up study. Mov Disord 2015;30:266-9.
70. Ascherio A, Schwarzschild MA. The epidemiology of Parkinson’s disease: risk factors and prevention. Lancet Neurol 2016;15:1257-72.
71. Reekes TH, Higginson CI, Ledbetter CR, Sathivadivel N, Zweig RM, Disbrow EA. Sex specific cognitive differences in Parkinson disease. NPJ Parkinsons Dis 2020;6:7.
72. Sullivan EV, Rosenbloom M, Serventi KL, Pfefferbaum A. Effects of age and sex on volumes of the thalamus, pons, and cortex. Neurobiol Aging 2004;25:185-92.
73. DeCarli C, Massaro J, Harvey D, et al. Measures of brain morphology and infarction in the framingham heart study: establishing what is normal. Neurobiol Aging 2005;26:491-510.
74. Ikram MA, Vrooman HA, Vernooij MW, et al. Brain tissue volumes in the general elderly population. The Rotterdam Scan Study. Neurobiol Aging 2008;29:882-90.
75. Salat DH, Tuch DS, Greve DN, et al. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol Aging 2005;26:1215-27.
76. Lemaître H, Crivello F, Grassiot B, Alpérovitch A, Tzourio C, Mazoyer B. Age- and sex-related effects on the neuroanatomy of healthy elderly. Neuroimage 2005;26:900-11.
77. Jäncke L, Mérillat S, Liem F, Hänggi J. Brain size, sex, and the aging brain. Hum Brain Mapp 2015;36:150-69.
78. Li W, van Tol MJ, Li M, et al. Regional specificity of sex effects on subcortical volumes across the lifespan in healthy aging. Hum Brain Mapp 2014;35:238-47.
79. Nemeth VL, Must A, Horvath S, Király A, Kincses ZT, Vécsei L. Gender-specific degeneration of dementia-related subcortical structures throughout the lifespan. J Alzheimers Dis 2017;55:865-80.
80. Wang Y, Xu Q, Luo J, Hu M, Zuo C. Effects of age and sex on subcortical volumes. Front Aging Neurosci 2019;11:259.
81. Goto M, Abe O, Miyati T, et al. Accelerated hippocampal volume reduction in post-menopausal women: an additional study with Atlas-based method. Radiol Phys Technol 2011;4:185-8.
82. Takahashi R, Ishii K, Kakigi T, Yokoyama K. Gender and age differences in normal adult human brain: voxel-based morphometric study. Hum Brain Mapp 2011;32:1050-8.
83. Good CD, Johnsrude I, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. Cerebral asymmetry and the effects of sex and handedness on brain structure: a voxel-based morphometric analysis of 465 normal adult human brains. Neuroimage 2001;14:685-700.
84. Luders E, Gaser C, Narr KL, Toga AW. Why sex matters: brain size independent differences in gray matter distributions between men and women. J Neurosci 2009;29:14265-70.
85. Cheng Y, Chou KH, Decety J, et al. Sex differences in the neuroanatomy of human mirror-neuron system: a voxel-based morphometric investigation. Neuroscience 2009;158:713-20.
86. Taki Y, Kinomura S, Sato K, Goto R, Kawashima R, Fukuda H. A longitudinal study of gray matter volume decline with age and modifying factors. Neurobiol Aging 2011;32:907-15.
87. Pfefferbaum A, Rohlfing T, Rosenbloom MJ, Chu W, Colrain IM, Sullivan EV. Variation in longitudinal trajectories of regional brain volumes of healthy men and women (ages 10 to 85 years) measured with atlas-based parcellation of MRI. Neuroimage 2013;65:176-93.
88. Jiang J, Sachdev P, Lipnicki DM, et al. A longitudinal study of brain atrophy over two years in community-dwelling older individuals. Neuroimage 2014;86:203-11.
89. Narvacan K, Treit S, Camicioli R, Martin W, Beaulieu C. Evolution of deep gray matter volume across the human lifespan. Hum Brain Mapp 2017;38:3771-90.
90. Vinke EJ, de Groot M, Venkatraghavan V, et al. Trajectories of imaging markers in brain aging: the Rotterdam Study. Neurobiol Aging 2018;71:32-40.
91. Ikram MA, Brusselle GGO, Murad SD, et al. The Rotterdam Study: 2018 update on objectives, design and main results. Eur J Epidemiol 2017;32:807-50.
92. Mattson MP, Arumugam TV. Hallmarks of brain aging: adaptive and Pathological modification by metabolic states. Cell Metab 2018;27:1176-99.
93. Rizzuto R, De Stefani D, Raffaello A, Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol 2012;13:566-78.
94. Demarest TG, McCarthy MM. Sex differences in mitochondrial (dys)function: Implications for neuroprotection. J Bioenerg Biomembr 2015;47:173-88.
95. Lloret A, Badía MC, Mora NJ, et al. Gender and age-dependent differences in the mitochondrial apoptogenic pathway in Alzheimer’s disease. Free Radic Biol Med 2008;44:2019-25.
96. Borrás C, Sastre J, García-sala D, Lloret A, Pallardó FV, Viña J. Mitochondria from females exhibit higher antioxidant gene expression and lower oxidative damage than males. Free Radic Biol Med 2003;34:546-52.
97. Guevara R, Santandreu FM, Valle A, Gianotti M, Oliver J, Roca P. Sex-dependent differences in aged rat brain mitochondrial function and oxidative stress. Free Radic Biol Med 2009;46:169-75.
98. Guevara R, Gianotti M, Oliver J, Roca P. Age and sex-related changes in rat brain mitochondrial oxidative status. Exp Gerontol 2011;46:923-8.
99. Gaignard P, Savouroux S, Liere P, et al. Effect of sex differences on brain mitochondrial function and its suppression by ovariectomy and in aged mice. Endocrinology 2015;156:2893-904.
100. Dkhil MA, Al-Shaebi EM, Lubbad MY, Al-Quraishy S. Impact of sex differences in brain response to infection with Plasmodium berghei. Parasitol Res 2016;115:415-22.
101. Khalifa AR, Abdel-Rahman EA, Mahmoud AM, et al. Sex-specific differences in mitochondria biogenesis, morphology, respiratory function, and ROS homeostasis in young mouse heart and brain. Physiol Rep 2017;5:e13125.
102. Ali SS, Xiong C, Lucero J, Behrens MM, Dugan LL, Quick KL. Gender differences in free radical homeostasis during aging: shorter-lived female C57BL6 mice have increased oxidative stress. Aging Cell 2006;5:565-74.
103. Viña J, Borrás C. Women live longer than men: understanding molecular mechanisms offers opportunities to intervene by using estrogenic compounds. Antioxid Redox Signal 2010;13:269-78.
104. Mandal PK, Tripathi M, Sugunan S. Brain oxidative stress: detection and mapping of anti-oxidant marker ‘Glutathione’ in different brain regions of healthy male/female, MCI and Alzheimer patients using non-invasive magnetic resonance spectroscopy. Biochem Biophys Res Commun 2012;417:43-8.
105. Rekkas PV, Wilson AA, Lee VW, et al. Greater monoamine oxidase a binding in perimenopausal age as measured with carbon 11-labeled harmine positron emission tomography. JAMA Psychiatry 2014;71:873-9.
106. Bellanti F, Matteo M, Rollo T, et al. Sex hormones modulate circulating antioxidant enzymes: impact of estrogen therapy. Redox Biol 2013;1:340-6.
107. Candeias E, Duarte AI, Sebastião I, et al. Middle-aged diabetic females and males present distinct susceptibility to Alzheimer disease-like pathology. Mol Neurobiol 2017;54:6471-89.
108. Sobocanec S, Balog T, Kusić B, Sverko V, Sarić A, Marotti T. Differential response to lipid peroxidation in male and female mice with age: correlation of antioxidant enzymes matters. Biogerontology 2008;9:335-43.
109. Silva TLA, Braz GRF, Silva SCA, et al. Serotonin transporter inhibition during neonatal period induces sex-dependent effects on mitochondrial bioenergetics in the rat brainstem. Eur J Neurosci ;2018:1620-34.
110. Cole TB, Coburn J, Dao K, et al. Sex and genetic differences in the effects of acute diesel exhaust exposure on inflammation and oxidative stress in mouse brain. Toxicology 2016;374:1-9.
111. Monte AS, Mello BSF, Borella VCM, et al. Two-hit model of schizophrenia induced by neonatal immune activation and peripubertal stress in rats: study of sex differences and brain oxidative alterations. Behav Brain Res 2017;331:30-7.
112. Al-Gubory KH, Garrel C. Sex-specific divergence of antioxidant pathways in fetal brain, liver, and skeletal muscles. Free Radic Res 2016;50:366-73.
113. Uzun H, Kayali R, Cakatay U. The chance of gender dependency of oxidation of brain proteins in aged rats. Arch Gerontol Geriatr 2010;50:16-9.
114. Yao J, Irwin R, Chen S, Hamilton R, Cadenas E, Brinton RD. Ovarian hormone loss induces bioenergetic deficits and mitochondrial β-amyloid. Neurobiol Aging 2012;33:1507-21.
115. Grimm A, Mensah-Nyagan AG, Eckert A. Alzheimer, mitochondria and gender. Neurosci Biobehav Rev 2016;67:89-101.
116. Serrano-Regal MP, Bayón-Cordero L, Ordaz RP, et al. Expression and function of GABA receptors in myelinating cells. Front Cell Neurosci 2020;14:256.
117. Bernaus A, Blanco S, Sevilla A. Glia crosstalk in neuroinflammatory diseases. Front Cell Neurosci 2020;14:209.
118. Augusto-Oliveira M, Arrifano GP, Takeda PY, et al. Astroglia-specific contributions to the regulation of synapses, cognition and behaviour. Neurosci Biobehav Rev 2020;118:331-57.
119. Thion MS, Low D, Silvin A, et al. Microbiome influences prenatal and adult microglia in a sex-specific manner. Cell 2018;172:500-516.e16.
120. Villa A, Gelosa P, Castiglioni L, et al. Sex-specific features of microglia from adult mice. Cell Rep 2018;23:3501-11.
121. Hanamsagar R, Alter MD, Block CS, Sullivan H, Bolton JL, Bilbo SD. Generation of a microglial developmental index in mice and in humans reveals a sex difference in maturation and immune reactivity. Glia 2017;65:1504-20.
122. Hayakawa N, Kato H, Araki T. Age-related changes of astorocytes, oligodendrocytes and microglia in the mouse hippocampal CA1 sector. Mech Ageing Dev 2007;128:311-6.
123. Mouton PR, Long JM, Lei D, et al. Age and gender effects on microglia and astrocyte numbers in brains of mice. Brain Res 2002;956:30-5.
124. Sierra A, Gottfried-Blackmore AC, McEwen BS, Bulloch K. Microglia derived from aging mice exhibit an altered inflammatory profile. Glia 2007;55:412-24.
125. Bonham LW, Sirkis DW, Yokoyama JS. The transcriptional landscape of microglial genes in aging and neurodegenerative disease. Front Immunol 2019;10:1170.
126. Mangold CA, Wronowski B, Du M, et al. Sexually divergent induction of microglial-associated neuroinflammation with hippocampal aging. J Neuroinflammation 2017;14:141.
127. Sala Frigerio C, Wolfs L, Fattorelli N, et al. The major risk factors for Alzheimer’s disease: age, sex, and genes modulate the microglia response to Aβ plaques. Cell Rep 2019;27:1293-1306.e6.
128. Kang SS, Ebbert MTW, Baker KE, et al. Microglial translational profiling reveals a convergent APOE pathway from aging, amyloid, and tau. J Exp Med 2018;215:2235-45.
129. Yanguas-Casás N, Crespo-Castrillo A, Arevalo MA, Garcia-Segura LM. Aging and sex: impact on microglia phagocytosis. Aging Cell 2020;19:e13182.
130. Del Moral M, Fröhlich N, Figarella K, Mojtahedi N, Garaschuk O. Effect of caloric restriction on the in vivo functional properties of aging microglia. Front Immunol 2020;11:750.
131. Chowen JA, Garcia-Segura LM. Role of glial cells in the generation of sex differences in neurodegenerative diseases and brain aging. Mech Ageing Dev 2021;196:111473.
132. Cerghet M, Skoff RP, Bessert D, Zhang Z, Mullins C, Ghandour MS. Proliferation and death of oligodendrocytes and myelin proteins are differentially regulated in male and female rodents. J Neurosci 2006;26:1439-47.
133. Cerghet M, Skoff RP, Swamydas M, Bessert D. Sexual dimorphism in the white matter of rodents. J Neurol Sci 2009;286:76-80.
134. Young KM, Psachoulia K, Tripathi RB, et al. Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron 2013;77:873-85.
135. Yasuda K, Maki T, Kinoshita H, et al. Sex-specific differences in transcriptomic profiles and cellular characteristics of oligodendrocyte precursor cells. Stem Cell Res 2020;46:101866.
136. Li WW, Penderis J, Zhao C, Schumacher M, Franklin RJ. Females remyelinate more efficiently than males following demyelination in the aged but not young adult CNS. Exp Neurol 2006;202:250-4.
137. Anisimova AS, Alexandrov AI, Makarova NE, Gladyshev VN, Dmitriev SE. Protein synthesis and quality control in aging. Aging (Albany NY) 2018;10:4269-88.
138. Raihan O, Brishti A, Li Q, et al. SFRS11 loss leads to aging-associated cognitive decline by modulating LRP8 and ApoE. Cell Rep 2019;28:78-90.e6.
139. Pluvinage JV, Haney MS, Smith BAH, et al. CD22 blockade restores homeostatic microglial phagocytosis in ageing brains. Nature 2019;568:187-92.
140. Zullo JM, Drake D, Aron L, et al. Regulation of lifespan by neural excitation and REST. Nature 2019;574:359-64.
141. Yuan J, Chang SY, Yin SG, et al. Two conserved epigenetic regulators prevent healthy ageing. Nature 2020;579:118-22.
142. Tsuji T, Shimohama S. Protein degradation in Alzheimer’s disease and aging of the brain. Prog Mol Subcell Biol 2002;29:43-60.
143. Galluzzi L, Baehrecke EH, Ballabio A, et al. Molecular definitions of autophagy and related processes. EMBO J 2017;36:1811-36.
144. VerPlank JJS, Goldberg AL. Regulating protein breakdown through proteasome phosphorylation. Biochem J 2017;474:3355-71.
145. Ding Q, Keller JN. Proteasomes and proteasome inhibition in the central nervous system. Free Radic Biol Med 2001;31:574-84.
146. Zeng BY, Medhurst AD, Jackson M, Rose S, Jenner P. Proteasomal activity in brain differs between species and brain regions and changes with age. Mech Ageing Dev 2005;126:760-6.
147. Jenkins EC, Shah N, Gomez M, et al. Proteasome mapping reveals sexual dimorphism in tissue-specific sensitivity to protein aggregations. EMBO Rep 2020;21:e48978.
148. Pomatto LCD, Wong S, Carney C, Shen B, Tower J, Davies KJA. The age- and sex-specific decline of the 20s proteasome and the Nrf2/CncC signal transduction pathway in adaption and resistance to oxidative stress in Drosophila melanogaster. Aging (Albany NY) 2017;9:1153-85.
149. Dulka BN, Trask S, Helmstetter FJ. Age-related memory impairment and sex-specific alterations in phosphorylation of the Rpt6 proteasome subunit and polyubiquitination in the basolateral amygdala and medial prefrontal cortex. Front Aging Neurosci 2021;13:656944.
151. Congdon EE. Sex differences in autophagy contribute to female vulnerability in Alzheimer’s disease. Front Neurosci 2018;12:372.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Yang J, Qu J, Ma H. Recent developments in understanding brain aging: sex differences, mechanisms, and implications in diseases. Ageing Neur Dis 2022;2:3. http://dx.doi.org/10.20517/and.2022.03
AMA Style
Yang J, Qu J, Ma H. Recent developments in understanding brain aging: sex differences, mechanisms, and implications in diseases. Ageing and Neurodegenerative Diseases. 2022; 2(1): 3. http://dx.doi.org/10.20517/and.2022.03
Chicago/Turabian Style
Yang, Jing, Jing Qu, Huan Ma. 2022. "Recent developments in understanding brain aging: sex differences, mechanisms, and implications in diseases" Ageing and Neurodegenerative Diseases. 2, no.1: 3. http://dx.doi.org/10.20517/and.2022.03
ACS Style
Yang, J.; Qu J.; Ma H. Recent developments in understanding brain aging: sex differences, mechanisms, and implications in diseases. Ageing. Neur. Dis. 2022, 2, 3. http://dx.doi.org/10.20517/and.2022.03
About This Article
Copyright
Data & Comments
Data

 Cite This Article 15 clicks
Cite This Article 15 clicks












Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.